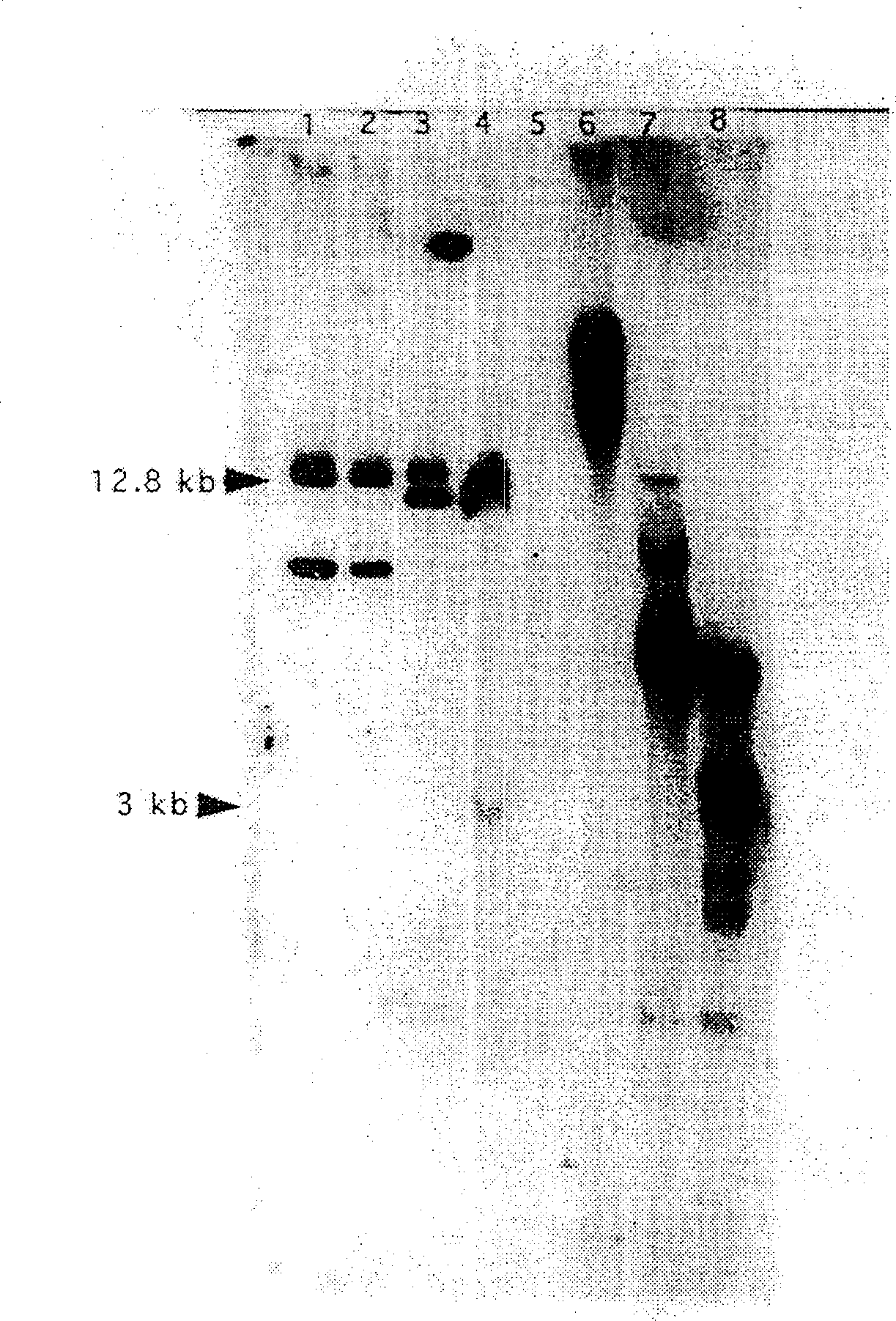
Genetically engineered duckweed
A duckweed and genetic technology, applied in genetic engineering, plant genetic improvement, gardening tools/equipment, etc., can solve problems such as system optimization of culture medium components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Eighteen combinations of the auxin 2,4-dichlorophenoxyacetic acid (2,4-D) and the cytokinin benzyladenine (BA) were tested for callus induction in the duckweed species Lemna gibba G3 role.
[0089] Before the experiment, duckweed leaves were cultured at 23°C for 2 weeks in liquid Hoagland medium (Hoagland and Snyder, Proc.Amer.Soc.Hort.Sci.30, 288 (1933)) containing 3% sucrose, photoperiod 16hr light / 8hr dark, the light intensity is about 40μmol / m 2 sec. To induce callus, prepare 18 aliquots of 100 ml Murashige and Skoog basal medium containing 3% sucrose, 0.15% Gelrite and 0.4% Difco Bacterial Agar with 2,4-D concentrations of 10, 20 and 50 μM, And the concentration of BA was 0, 0.01, 0.1, 1.0, 2.0 and 10.0 μM. The pH of all culture media was adjusted to 5.8, autoclaved at 121° C. for 20 minutes, cooled, and each 100 ml was poured into four 100 mm×15 mm petri dishes.
[0090] A full factorial experimental design of 3 2,4-D concentrations × 6 BA concentrations was u...
Embodiment 2
[0093] 40 concentrations of the auxin 2,4-dichlorophenoxyacetic acid (2,4-D) and the cytokinin benzyladenine (BA) were tested to optimize the auxin and cytokinin concentrations by Induced callus from duckweed leaves of Lemnagibba G3.
[0094] Before the experiment, duckweed leaves were cultured at 23°C for 2 weeks in liquid Hoagland medium containing 3% sucrose, with a photoperiod of 16 hr light / 8 hr dark and a light intensity of about 40 μmol / m 2 sec. To induce callus, prepare 40 aliquots of 100 ml Murashige and Skoog medium containing 3% sucrose, 0.15% Gelrite and 0.4% Difco Bacterial Agar with 2,4-D concentrations of 20, 30, 40, 50 , 60, 70, 80, 100 μM, while the concentrations of BA were 0.01, 0.05, 0.1, 0.5 and 1.0 μM. The pH of all culture media was adjusted to 5.8, autoclaved at 121° C. for 20 minutes, cooled, and each 100 ml was poured into four 100 mm×15 mm petri dishes.
[0095] A full factorial experimental design of 8 2,4-D concentrations × 5 BA concentrations w...
Embodiment 3
[0098] Forty combinations of the auxin dicamba and the cytokinin benzyladenine (BA) were tested to compare the relative efficacy of dicamba versus 2,4-D for callus induction of duckweed species Lemna gibba G3.
[0099] Before the experiment, duckweed leaves were cultured at 23°C for 2 weeks in liquid Hoagland medium containing 3% sucrose, with a photoperiod of 16 hr light / 8 hr dark and a light intensity of about 40 μmol / m 2 sec. To induce callus, prepare 40 aliquots of 100 ml Murashige and Skoog medium containing 3% sucrose, 0.15% Gelrite and 0.4% Difco Bacterial Agar with dicamba concentrations of 10, 20, 30, 40, 50, 60, 80, 100 μM, while the concentrations of BA were 0.01, 0.05, 0.1, 0.5 and 1.0 μM. The pH of all culture media was adjusted to 5.8, autoclaved at 121° C. for 20 minutes, cooled, and each 100 ml was poured into four 100 mm×15 mm petri dishes.
[0100] A full factorial experimental design of 8 dicamba concentrations x 5 BA concentrations was used (40 treatments...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com