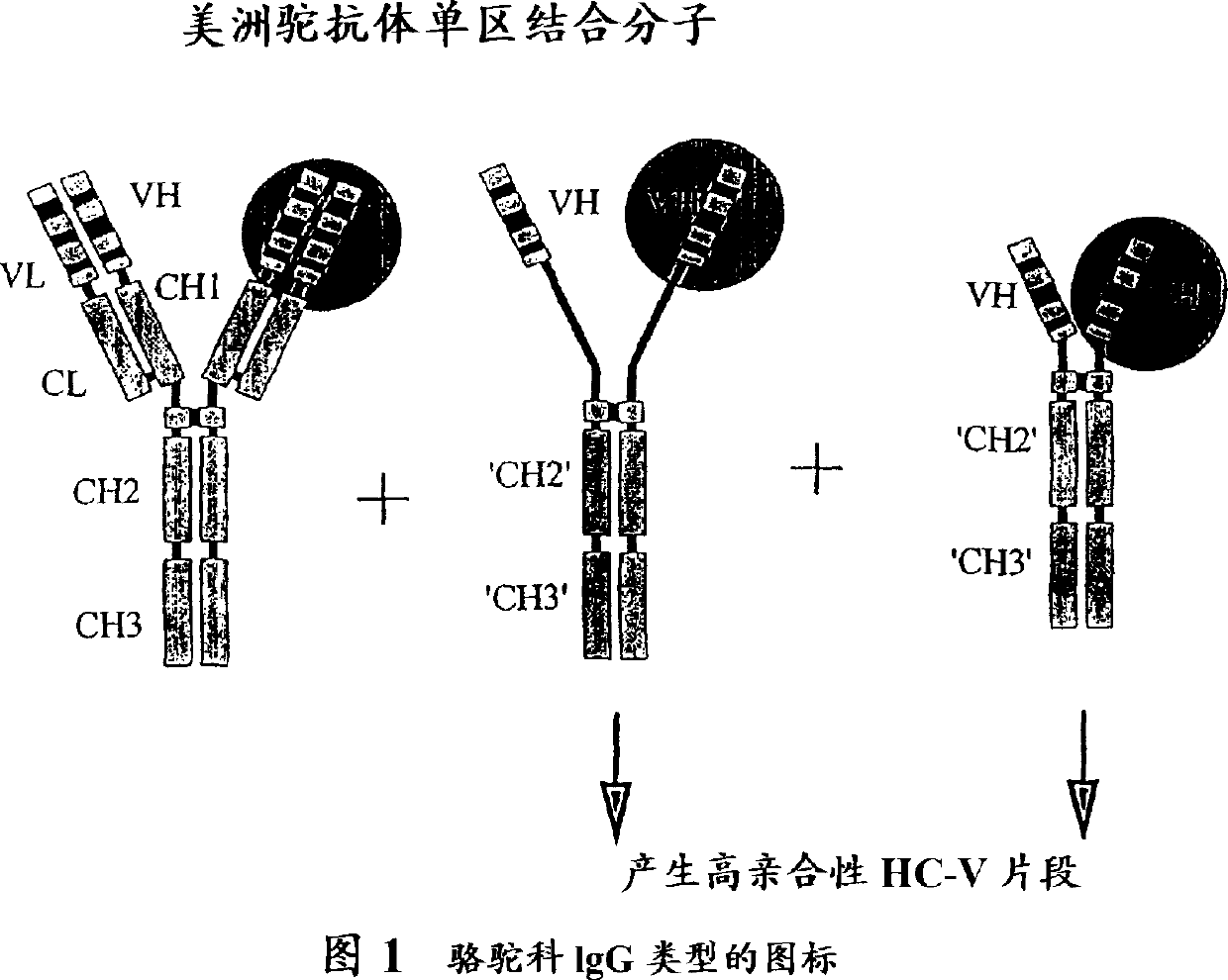
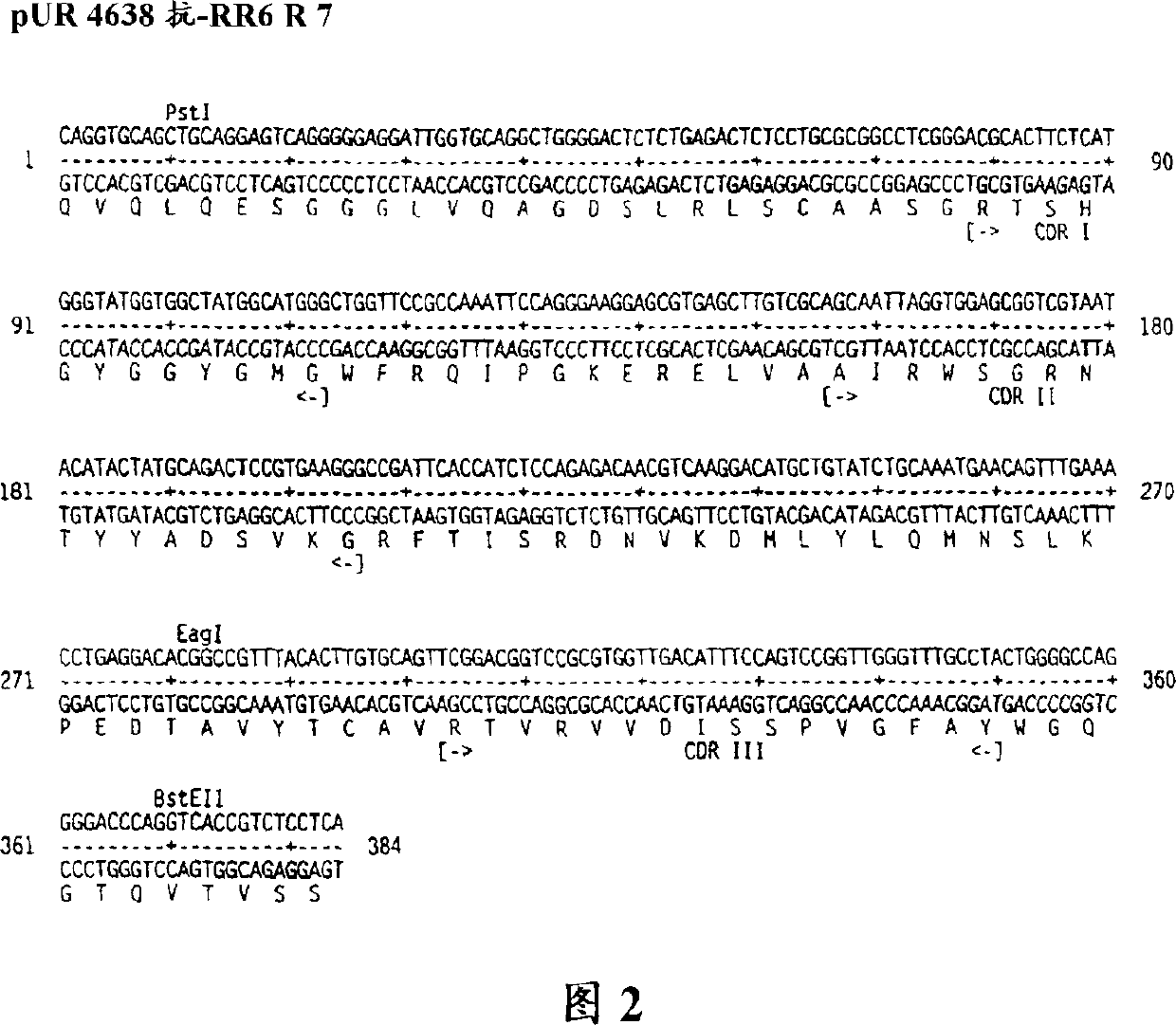
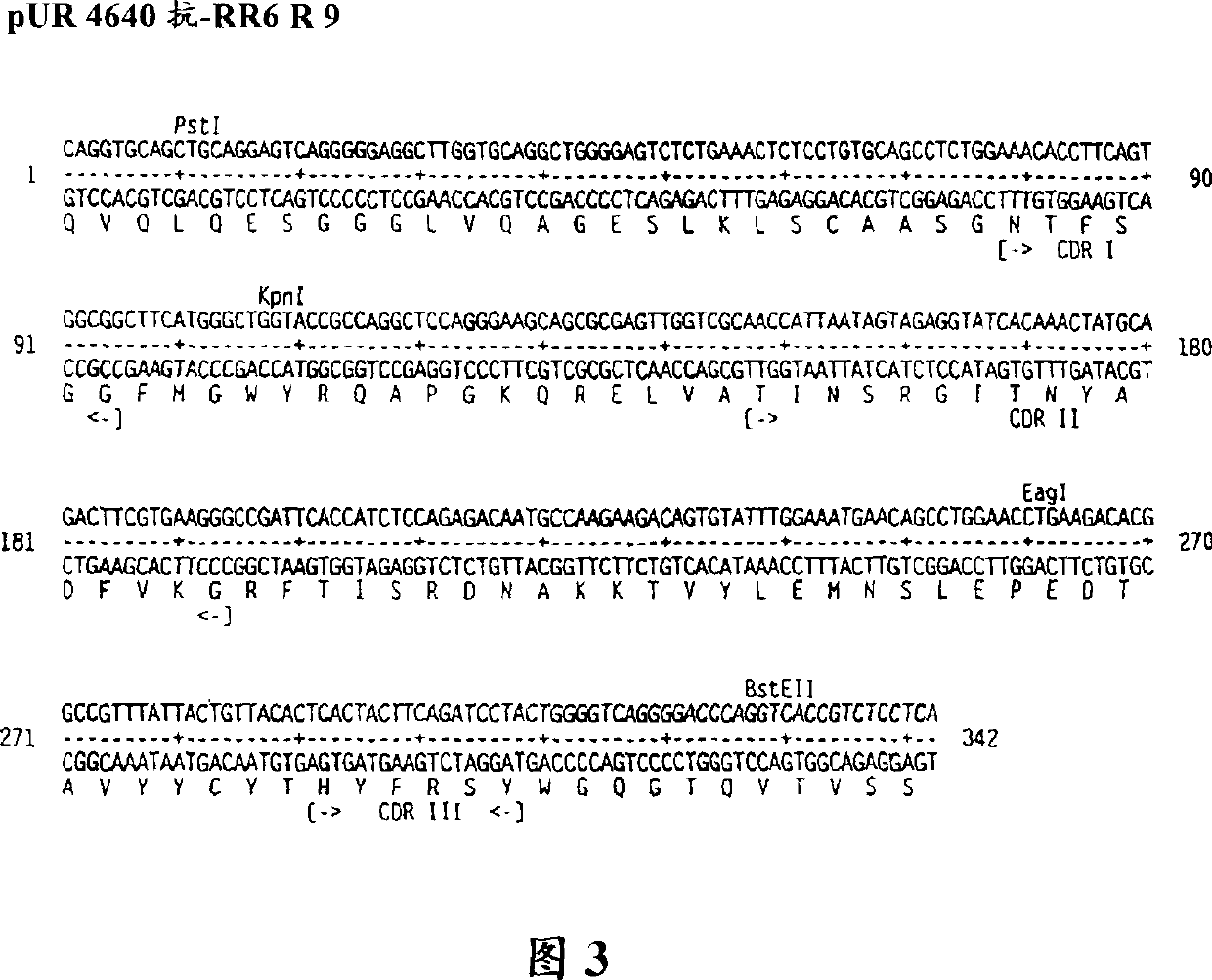
Multivalent antigen-binding proteins
A technology for binding proteins and multivalent antigens, applied in the direction of hybrid immunoglobulin, anti-bacterial immunoglobulin, anti-hormonal immunoglobulin, etc., can solve problems such as obstacles and affecting binding activity, and achieve small size, economy and effectiveness The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1. Induced humoral immune response in llamas
[0075] Male llamas were immunized subcutaneously and intramuscularly with water-in-oil emulsion (1:9 V / V, antigen in water: Specol (Bokout et al.)). 0.75-1.5 ml water-in-oil emulsion per immunization site, vaccination contains: 100 g antigen. Antigens used were: hCG (Sigma), azo-stained RR6 (ICI) linked to BSA via a reactive triazine group, and Streptococcus mutant HG982 cells. Immunizations were given according to the following schedule: the second immunization three weeks after the first immunization. The third time was performed two weeks after the second time. Antigen-specific ELISAs track the immune response.
[0076] Anti-RR6 responses were measured using Nunc Covalink plates, which were stained with azo dyes. After incubation with (diluted) serum samples, they were incubated with polyclonal rabbit-anti-llama antiserum (obtained by immunizing rabbits with llama antibodies conjugated to llama immunoglobulin...
Embodiment 2
[0077] Cloning, expression and screening of embodiment 2 llama HC-V fragments
[0078] 2.1 Isolation of gene fragments encoding llama HC-V region
[0079] 200 ml of blood samples from immunized llamas were centrifuged to obtain enriched lymphocyte populations by step centrifugation in Ficoll (Pharmacia), and total RNA was isolated by acid guanidinium thiocyanate extraction (for example, by the method of Chomczynnski and Sacchi, 1987). After synthesizing first-strand cDNA (for example, with the Amersham First-Strand cDNA Kit), PCR amplify the DNA fragment encoding the HC-V segment and part of the long or short hinge region using special primers:
[0080] PstIVH-2B 5'-AGGTSMAR CTGCAG SAGTCWGG-3' (see SEQ.ID.NO:2) S=C and G, M=A and C, R=A and G, W=A and T,
[0081] Hind IIILam-075'-AACAGTT AAGCTT CCGCTTGCGGCCGCGGAGCTGGGGTCTTCGCTGTGGTGCG-3'
[0082] (short hinge) (see SEQ.ID.NO: 3)
[0083] Hind IIILam-0...
Embodiment 3
[0129] Example 3 Preparation of llama HC-V double heads by Saccharomyces cerevisiae
[0130] 3.1 Construction of episomal expression plasmid encoding anti-hCG / anti-RR6 bispecific double head
[0131] In the anti-hCG HC-V fragments H14 and HI15 (anti-α-subunit), the PstI site was removed and the XhoI site was introduced by PCR using the following primers: °C
[0132] MPG158WB
[0133] XhoI5'-GAATTAAGCGGCCGCCCAGGTGAAACTG CTCGAG TCWGGGGGA-3' (see SEQ.ID.NO: 27) and MPG159WB
[0134] BstEII 3'-CCCTGGGT CCAGTGG CAGAGGAGTGGCAGAGGAGTCTTGTTT-5' (see SEQ.ID.NO: 28) sequence in this way:
[0135] PstICAG GTC CAG CTG CAG GAG TCT GGG Q V Q L Q E S G (see SEQ.ID.NO:29 and NO:30) becomes
[0136] XhoICAG GTG AAA CTG CTC GAG TCW GGG Q V K L L E S G (see SEQ.ID.NO:31 and NO:32)
[0137] The PCR fragment was digested with XhoI and BstEII, and the 330bp fragment was purified by agarose gel electrophoresis and separated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com