Method of producing human forcing erythrogenin using transgene animal mammary gland
An erythropoietin, mammary gland technology, applied in the field of genetic engineering and transgenic animals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Construction of EPO mammary gland expression vector
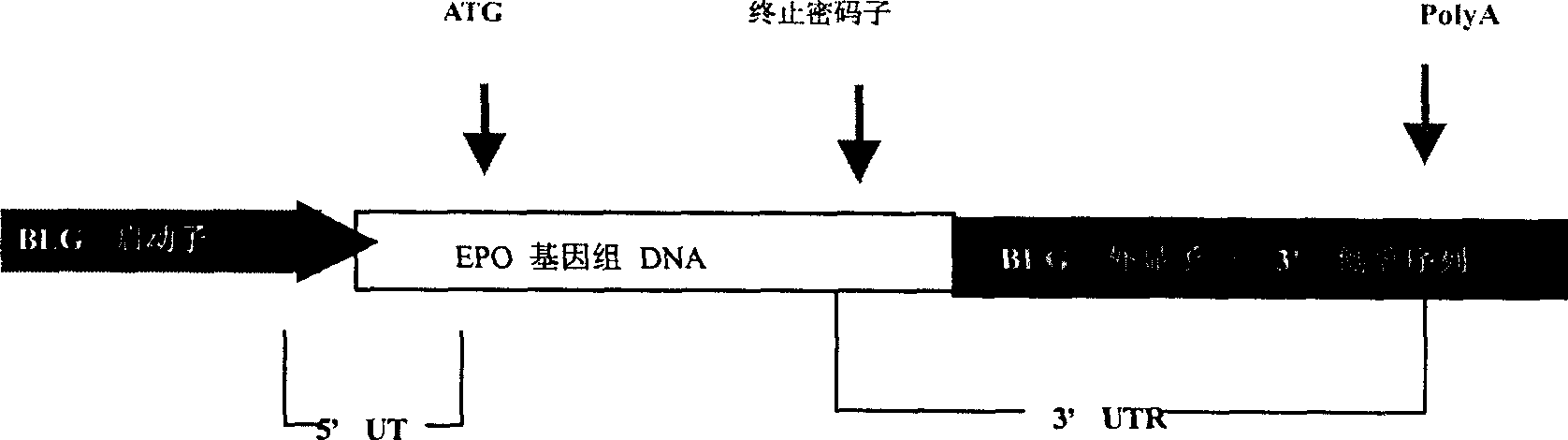
[0043] The gene construction process of this embodiment is as follows: figure 2 As shown, the gene construct is a BLG-EPO fusion gene, wherein the regulatory sequence is derived from bovine BLG and EPO, and the coding sequence is derived from EPO.
[0044] Preparation of pBLG-EPO
[0045] 1. The source of each component
[0046] 1) Bovine BLG 5' flanking sequence and exon 1 non-coding sequence
[0047] According to the sequence reported by GENBANK X14710, the following primers were designed and synthesized: 5'-cggccgggggtctgctcc-3' (SEQ ID NO: 5) and 5'-ctcgagggctgcagctggggtcac-3' (SEQ ID NO: 6), using bovine genomic DNA as a template, using PCR Method (94°C, 5min; 94°C, 1min; 60°C, 45s; 72°C, 90s, 30 cycles) to amplify the BLG5' flanking sequence and the non-coding part of exon 1 (SEQ ID NO: 1). The PCR product was cloned into pGEM-Teasy vector (Promega Company) to obtain plasmid pBLG5'.
[0048] 2) ...
Embodiment 2
[0065] Example 2 Preparation of fusion gene pBLG-EPO transgenic goat
[0066] 1. Preparation of plasmid DNA for transgenesis
[0067] 1) Transform Escherichia coli DH52 with the plasmid pBLG-EPO, culture the DH52 strain containing the pBLG-EPO plasmid in liquid, and the culture volume can be 100ml-1000ml according to needs, then collect the bacteria, and prepare pBLG- by rapid extraction method or large-scale extraction method EPO (refer to the Molecular Cloning Handbook edited by Maniantis for detailed operation).
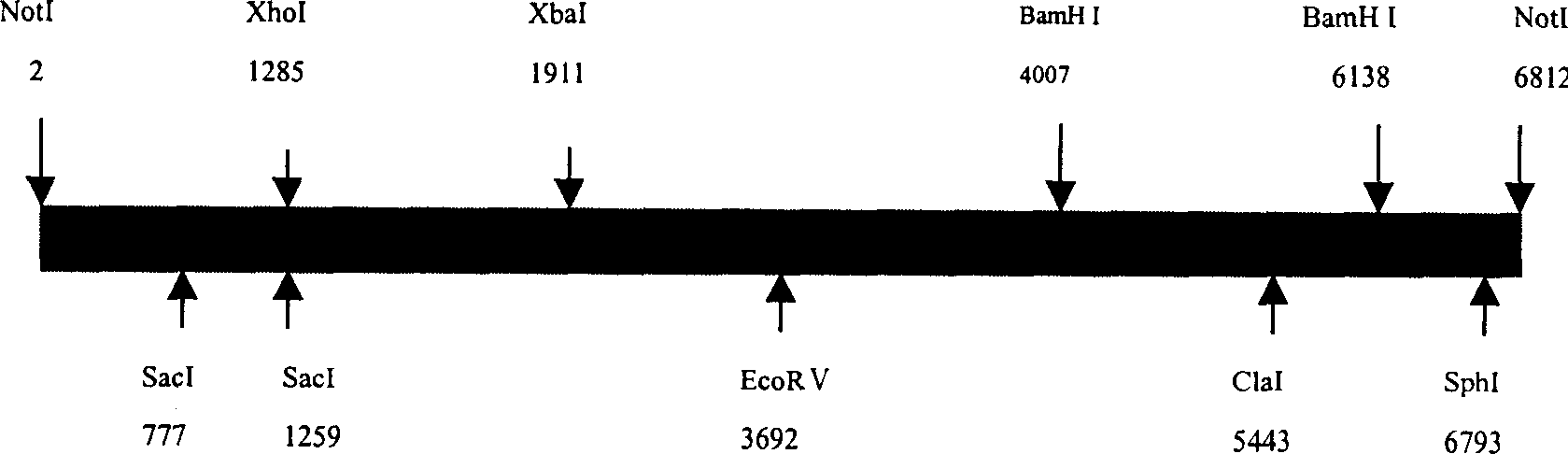
[0068] The extracted pBLG-EPO plasmid was digested with NotI, subjected to agarose gel electrophoresis, and a 6.82Kb fragment was recovered and purified with a SephaglassBabndprep (pharmacia biotech) kit for future use.
[0069] 2) Dilute the purified DNA to 2ug / ml with microinjection DNA diluent, centrifuge (12000rpm, 30min), aliquot and use for microinjection.
[0070] 2. Preparation of Transgenic Animals
[0071] The fusion gene BLG-EPO was introduced into t...
Embodiment 3
[0072] Example 3. Detection of fusion gene pBLG-EPO transgenic goat genome integration
[0073] 1. Sample Processing
[0074] For non-transgenic goats and transgenic goats to be tested, ears (less than 1 cm in length) were taken according to routine operations, and stored at -20°C for later use.
[0075] 2. Detection method
[0076] 1) Rapid extraction of genomic DNA: put sheep ears in a 1.5ml centrifuge tube, add 0.5ml LysisBuffer (4M Urea power, 10mM EDTA (pH8.0), 0.5% Sarkosyl (Sigma L5125), 0.1MTris- Cl (pH 8.0), 0.2M NaCl), 50 μl of proteinase K (10 mg / ml) were placed in a hybridization oven at 55° C. and shaken overnight. Centrifuge (14000rpm) for 5 minutes, transfer the supernatant to a clean centrifuge tube, add 1ml of absolute ethanol to mix gently, shake vigorously, use Tip to gently stir up flocculent DNA precipitate, and use 250-300μl of 0.1×TE Or resuspend the DNA in double distilled water, mix by pipetting, and store at 4°C.
[0077] 2) Digestion and electrop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com