Process for the production of ultrafine powders
A technology of ultra-fine powder and manufacturing method, which is applied in chemical instruments and methods, oxide/hydroxide preparation, alumina/hydroxide preparation, etc., and can solve problems such as limitation and long-time grinding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 - by Ce(OH) 4 Synthesis of ultrafine CeO 2 particles
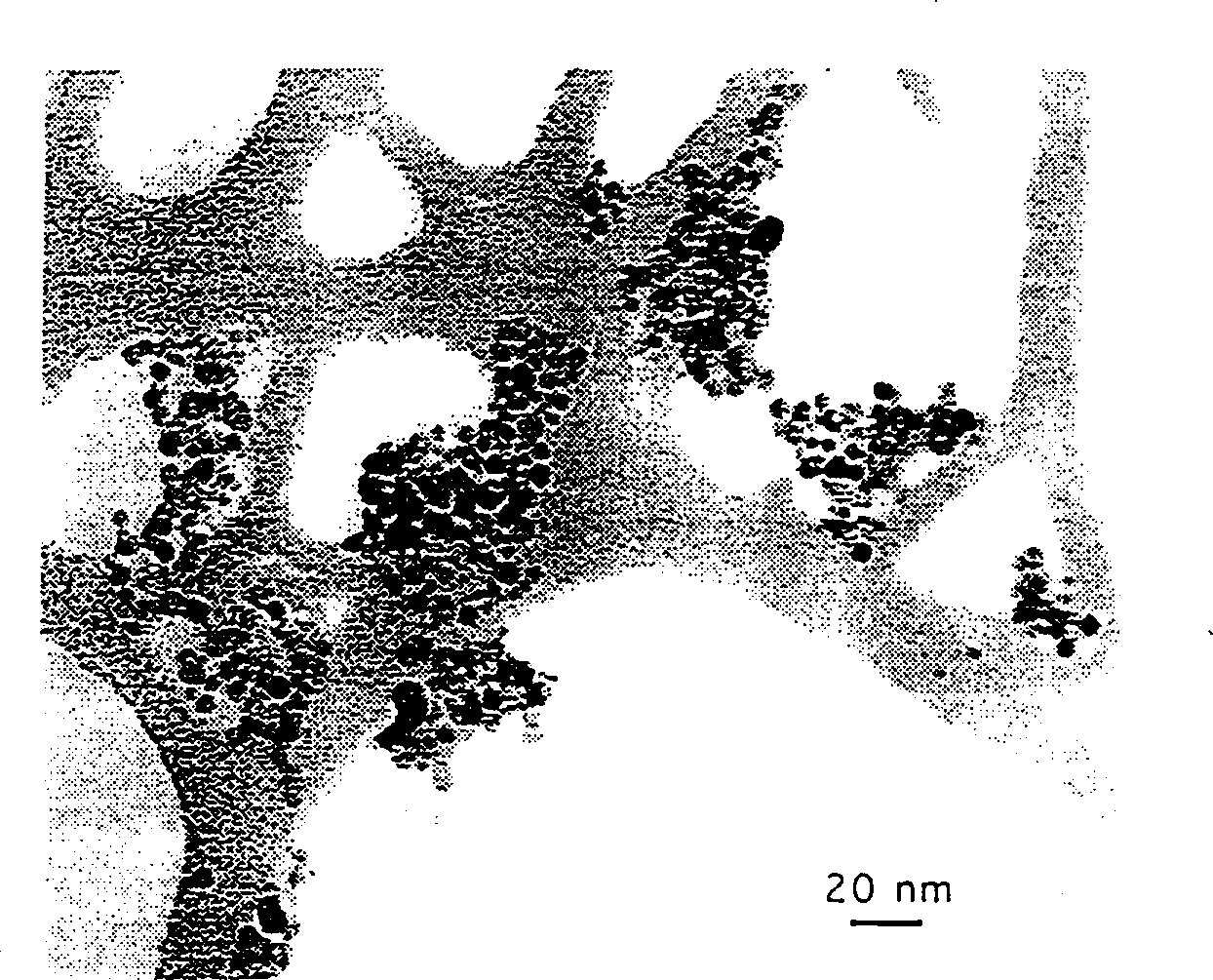
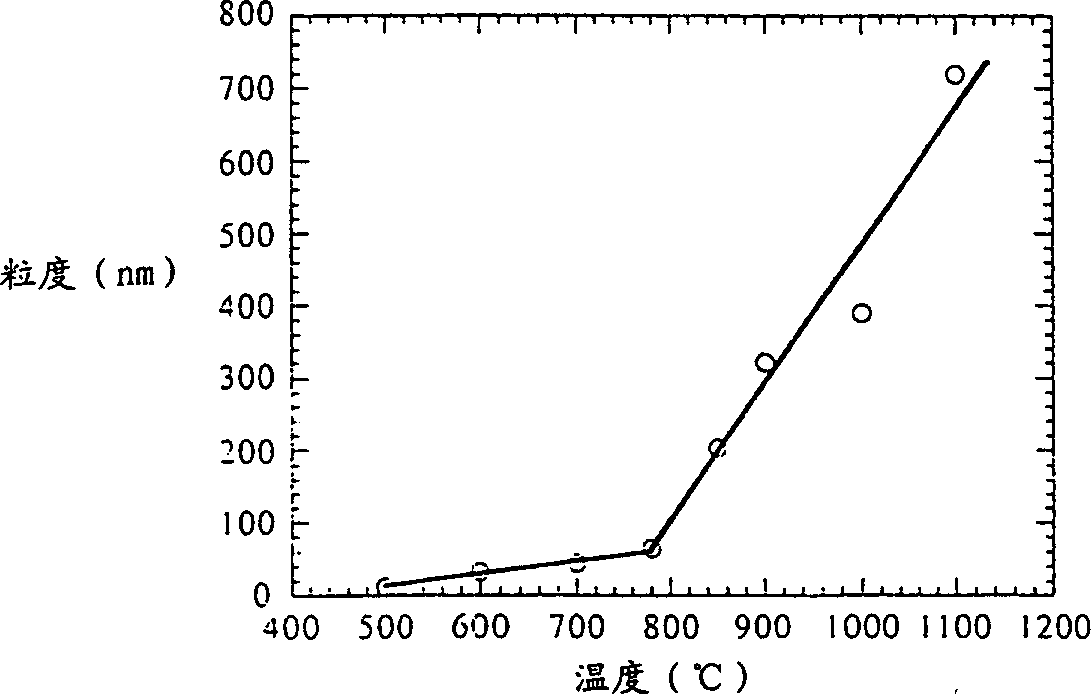
[0049] The material used is Ce(OH) 4 (97%, -100 mesh) and NaCl (>99.5%, ≤500 microns). Under the air atmosphere, add 28.4% (weight) Ce(OH) 4 Ce(OH) 4 Raw material mixture with NaCl powder, and sealed, the ratio of the two raw materials is equivalent to Ce(OH) 4 +9NaCl. The mass ratio of spheres to added powder was 40:1. Grind for 1-10 hours on a SPEX 8000 mixer / mill. After grinding, the powder was calcined at 500° C. for 1 hour in air. Wash the powder with distilled water using an ultrasonic bath and centrifuge to remove NaCl. The washed powder was evaporated and dried at 60°C in air. CeO 2 The particle size is measured by X-ray diffraction, transmission electron microscopy (TEM) and BET surface area to be 10-30 nm. figure 1 Representative nanoparticles of samples after milling for 6 h are shown.
[0050] In the second experiment, a 1 liter capacity grinder was used instead of the SPEX grinder...
Embodiment 2
[0052] Example 2 - by SnCl 2 Synthetic SnO 2
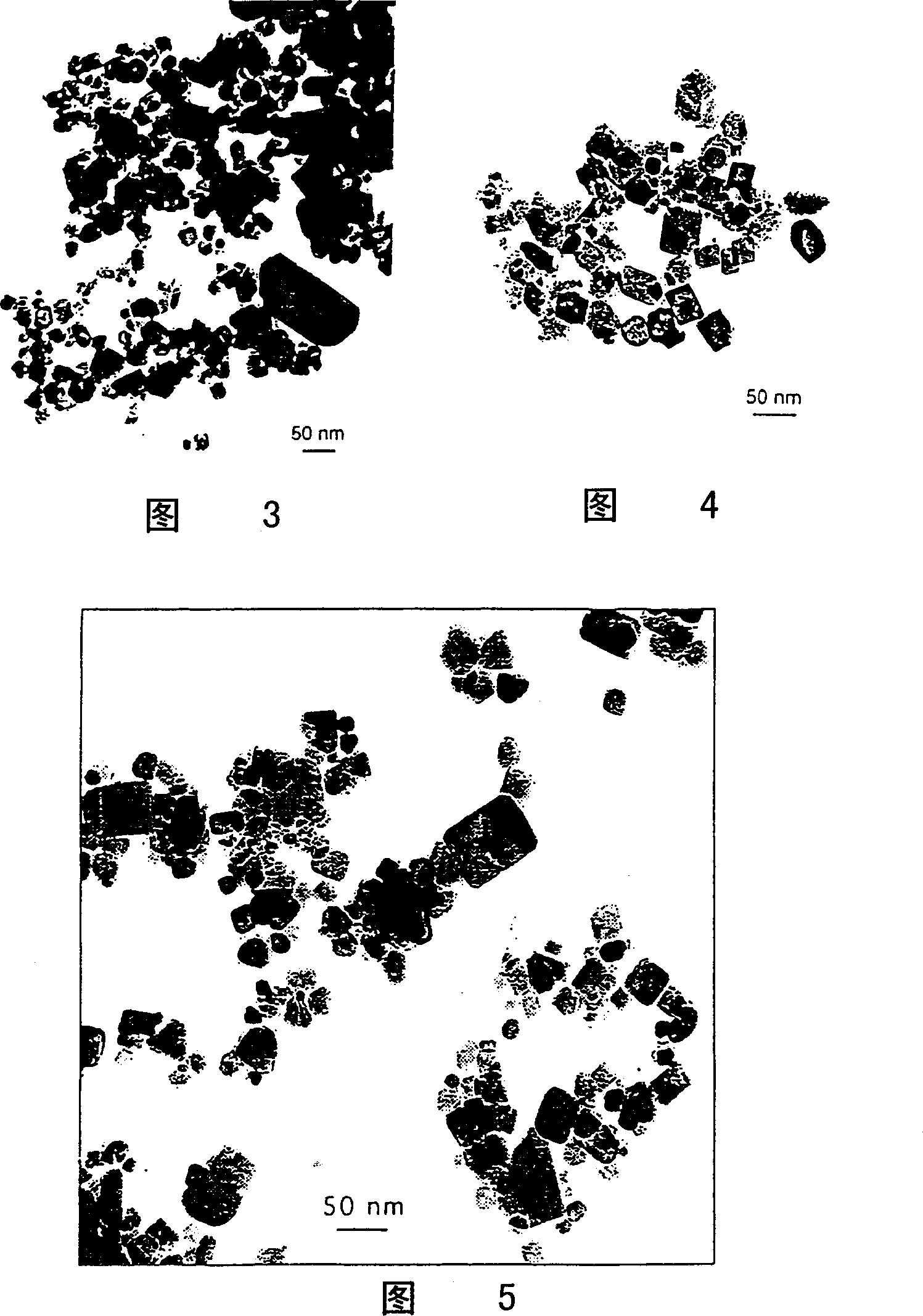
[0053] The material used is SnCl 2 (>99%) and NaCl (>99.5%). In an argon atmosphere, add SnCl in a SPEX mixer / mill equipped with 50 g of 6.4 mm diameter steel grinding media 2 The starting mixture with NaCl powder has a total mass of 5 g and a volume ratio of 1:10. The mass ratio of balls to powder was 10:1. Grinding was carried out for 3 hours. After grinding, the powder was heated at 800 °C for 30 min in an air atmosphere to make the SnCl 2 oxidation. The powder was then washed with distilled water to remove the NaCl diluent. The washed powder was dried in an oven at 60°C. Obtaining isolated equiaxed SnO 2 Nanoparticles, whose particle size is 20-200 nanometers, have many small crystal faces on the particle surface. Figure 3 shows the SnO formed after heat treatment 2 Transmission electron micrograph (TEM) of the particles.
Embodiment 3
[0054] Example 3 - by Al(OH) 3 Synthetic Al 2 o 3
[0055] The material used is Al(OH) 3 (-100 mesh) and NaCl (>99.5%, ≤500 microns). In a nitrogen atmosphere, a 7-liter mill containing 25 kg of stainless steel balls with a diameter of 6 mm was added containing 9% (by weight) Al(OH) 3 (corresponding to 10% by volume of Al(OH) 3 ) of Al(OH) 3 Mix the stock with NaCl powder and seal. The mass ratio of spheres to added powder was 22:1. Grind for 2 hours. After grinding, the powder was calcined at 850° C. for 1 hour in air. Using an ultrasonic bath and centrifuge, the powder was washed with deionized water to remove NaCl. The washed powder was evaporated and dried at 60°C in air. X-ray diffraction measurements show that during heat treatment Al(OH) 3 Dehydration forms gamma alumina. Al formed 2 o 3 The particle size was 11 nm as measured by BET surface area.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com