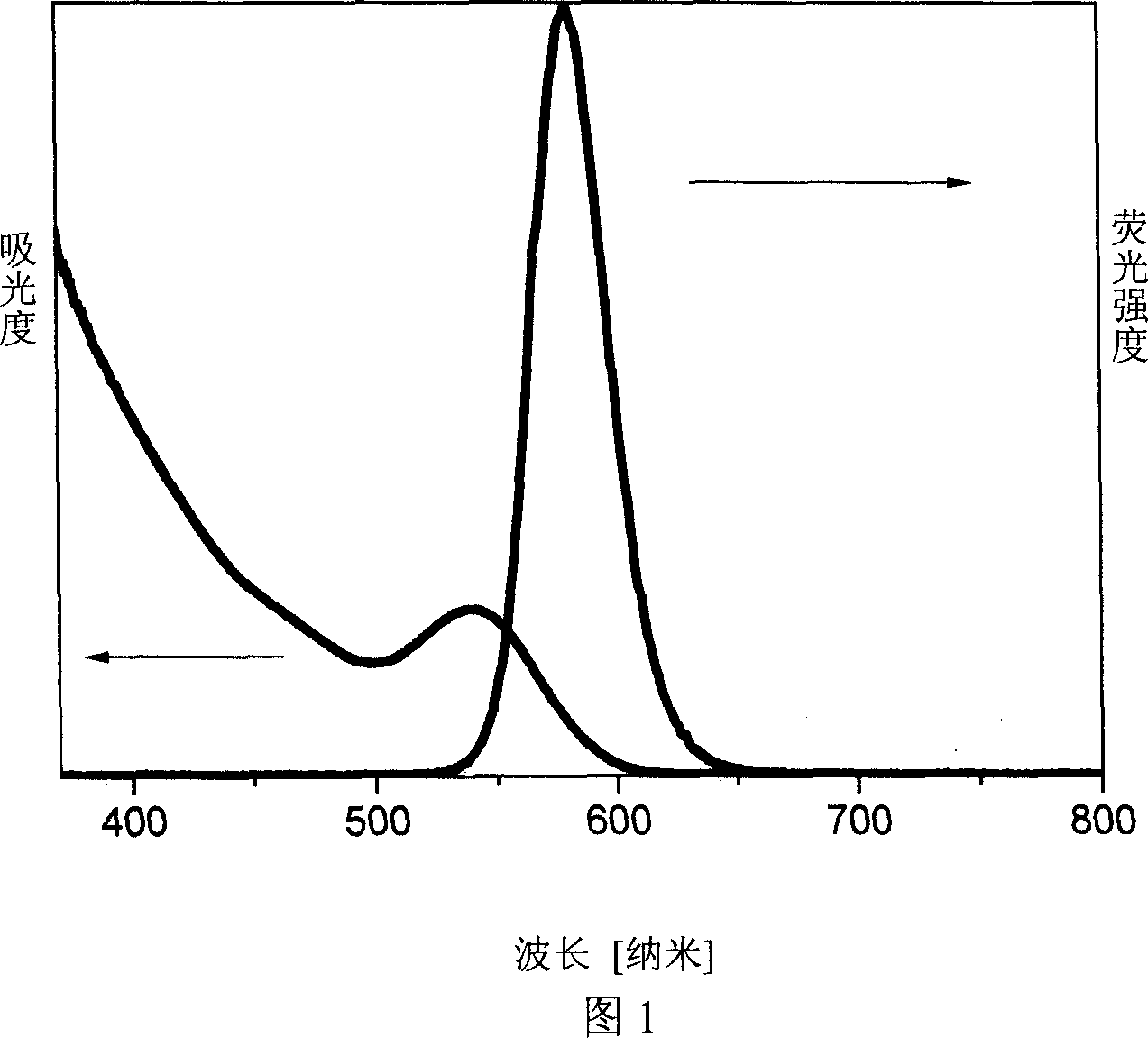
Process for preparing water soluble CdTe/CdS nuclear/shell type quantum point by microwave radiation reaction
A water-soluble, quantum dot technology, applied in chemical instruments and methods, cadmium compounds, inorganic chemistry, etc., can solve the problems of long time (a few days to dozens of days, etc., achieve good water solubility, good stability, and easy raw materials) the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1). Preparation of sodium telluride hydride:
[0019] 90.5 mg NaBH 4 Put the solid and 91.2 mg of Te powder into a small flask, add 3 ml of water, and react at 10 degrees Celsius for 10 hours to obtain a NaHTe solution for later use;
[0020] (2) Preparation of CdTe quantum dots
[0021] 22.5 mg CdCl 2 Dissolve in 100 ml of water, add 0.03 ml of mercaptoacetic acid, adjust pH=9.5 with 0.5 mol / L NaOH solution, pass nitrogen gas for 30 minutes, heat up to 100 degrees Celsius, inject 0.2 ml of NaHTe solution, and react for 3 hours to obtain CdTe quantum dot solution ;
[0022] (3) Preparation of CdTe / CdS precursor solution
[0023] 18.5 mg CdCl 2 and 5.6 mg Na 2 Dissolve S in 100 ml of water, add 0.06 ml of thioglycolic acid, adjust the pH to 10.5 with 0.5 mol / L NaOH, inject 10 ml of CdTe quantum dot solution, and obtain a CdTe / CdS precursor solution;
[0024] (4) Preparation of CdTe / CdS core / shell quantum dots by microwave irradiation
[0025] The obtained CdTe / C...
Embodiment 2
[0028] (1). Preparation of sodium telluride hydride
[0029] 90.7 mg NaBH 4 Put the solid and 127.6 mg of Te powder into a small flask, add 2.5 ml of water, and react at 0 degrees Celsius for 8 hours to obtain a NaHTe solution for subsequent use;
[0030] (2) Preparation of CdTe quantum dots
[0031] 30.0 mg CdCl 2 Dissolve in 100 ml of water, add 0.05 ml of mercaptoacetic acid, adjust the pH to 9.0 with 0.5 mol / L NaOH solution, pass nitrogen gas for 30 minutes, heat up to 100 degrees Celsius, inject 0.25 ml of NaHTe solution, and react for 5 hours to obtain a CdTe quantum dot solution ;
[0032] (3) Preparation of CdTe / CdS precursor solution
[0033] 25.9 mg CdCl 2 and 3.9 mg Na 2 Dissolve S in 100 ml of water, add 0.03 ml of mercaptoacetic acid, adjust pH=8 with 0.5 mol / L NaOH solution, inject 20 ml of CdTe quantum dot solution, and obtain a CdTe / CdS precursor solution;
[0034] (4) Preparation of CdTe / CdS core / shell quantum dots by program-controlled microwave irradi...
Embodiment 3
[0038] (1). Preparation of potassium hydride telluride
[0039] 50.8 mg KBH 4 Put the solid and 63.8 mg of Te powder into a small flask, add 2 ml of water, and react at 20 degrees Celsius for 15 hours to obtain a KHTe solution for later use;
[0040] (2) Preparation of CdTe quantum dots
[0041] 25.6 mg CdCl 2 Dissolve in 100 ml of water, add 0.018 ml of mercaptoacetic acid, adjust pH=8.5 with 0.5 mol / L NaOH solution, pass nitrogen gas for 30 minutes, heat up to 90 degrees Celsius, inject 0.3 ml of KHTe solution, and react for 10 hours to obtain CdTe quantum dot solution ;
[0042](3) Preparation of CdTe / CdS precursor solution
[0043] 15.5 mg CdCl 2 and 5.0 mg Na 2 Dissolve S in 100 ml of water, add 0.02 ml of thioglycolic acid, adjust the pH to 9.5 with 0.5 mol / L NaOH solution, and inject 15 ml of CdTe quantum dot solution to obtain a CdTe / CdS precursor solution;
[0044] (4) Preparation of CdTe / CdS core / shell quantum dots by program-controlled microwave irradiation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com