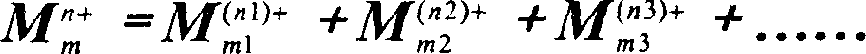Crystalline aluminosilicate zeolitic composition: UZM-4M, preparing method thereof and use in hydrocarbon conversion process and molecule substance mixture isolation
A technology of microporous crystalline zeolite and mixture, applied in the field of aluminosilicate zeolite, can solve the problems such as easy damage of zeolite Q framework
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Synthesis of UZM-4
[0091] In a beaker, 1305.6 grams of 35% by weight aqueous tetraethylammonium hydroxide (TEAOH) was mixed with 75.6 grams of aluminum hydroxide and stirred until dissolved. To this solution was added 331.2 grams of deionized (DI) water followed by the slow addition of 287.6 grams of Ludox TM AS-40. The resulting reaction mixture was stirred at room temperature for 2 hours, added in portions to a 1 liter Teflon bottle, and the bottle was placed in an oven at 95°C for 24 hours, then cooled to room temperature to form the aluminosilicate reaction mixture.
[0092] In a small beaker 13.3 grams of solid lithium chloride were combined with 68.0 grams of solid tetramethylammonium chloride (TMACl) and mixed with enough DI water to form a homogeneous solution. This aqueous solution was then slowly added dropwise with vigorous mixing to 1600 grams of the aluminosilicate reaction mixture. When the addition was complete, the resulting mixture was homogenized ...
Embodiment 2
[0094] Exchange of UZM-4
[0095] 1.0 g NH per 5.7 g deionized water in a glass beaker 4 NO 3 ratio of NH 4 NO 3 Mix with deionized water to prepare NH 4 NO 3 Exchange solution. The UZM-4 of Example 1 was added to the solution at a ratio of 1 gram of UZM-4 per gram of ammonium nitrate used in the solution. The slurry was heated to 80°C for 1 hour, then filtered and washed with warm (50°C) deionized water. Repeat this exchange step two more times. After the third exchange the UZM-4 product was washed with deionized water, dried at 50°C for 16 hours and rehydrated at ambient conditions for 24 hours. Chemical analysis showed lithium content ranging from 5.40% by weight (Li 2 0% by weight without volatile components) to 0.19% by weight. The amount of carbon also decreased from 8.90 wt% to 0.39 wt%, indicating the removal of the organic template.
Embodiment 3
[0097] AFS processing of UZM-4A
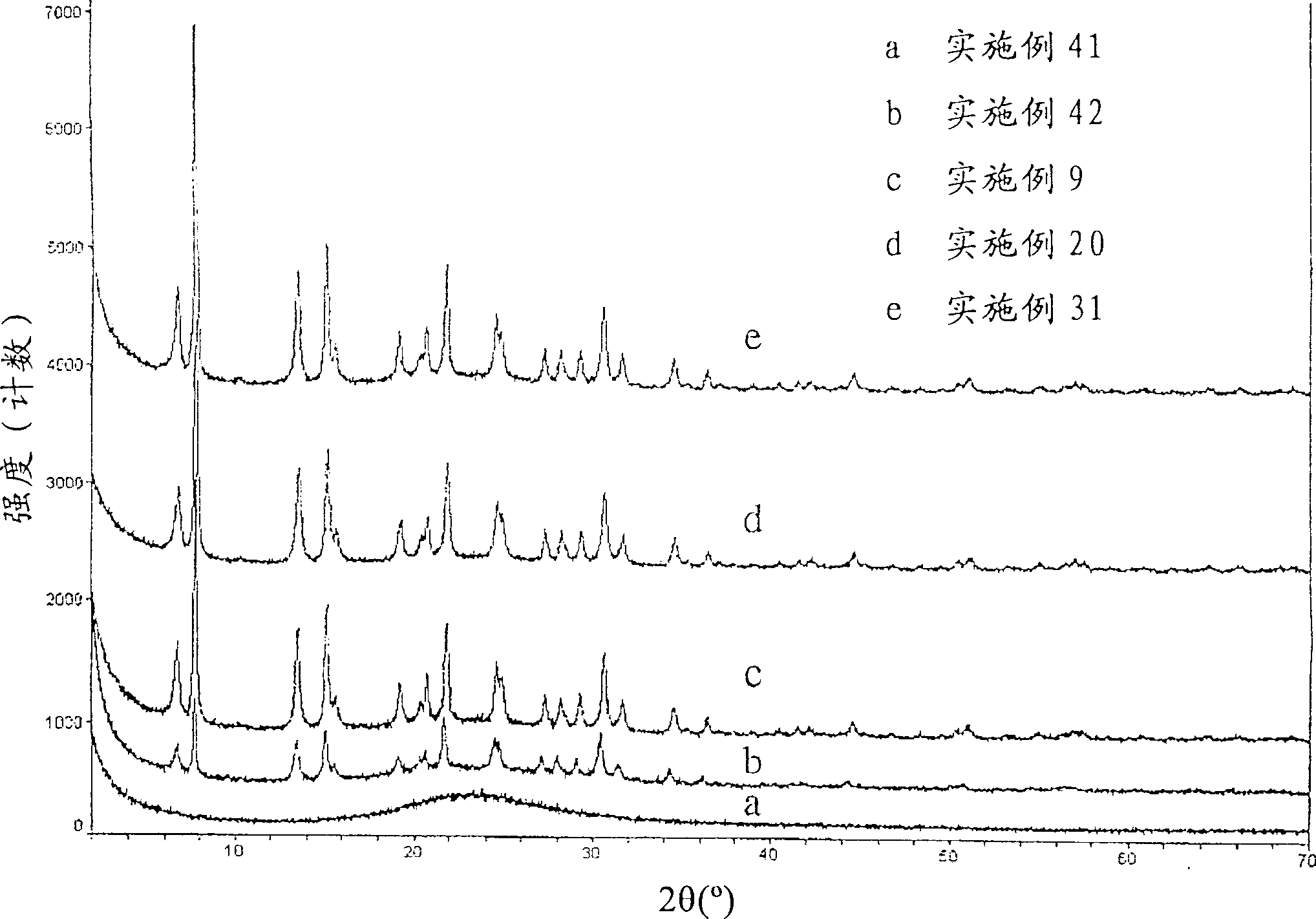
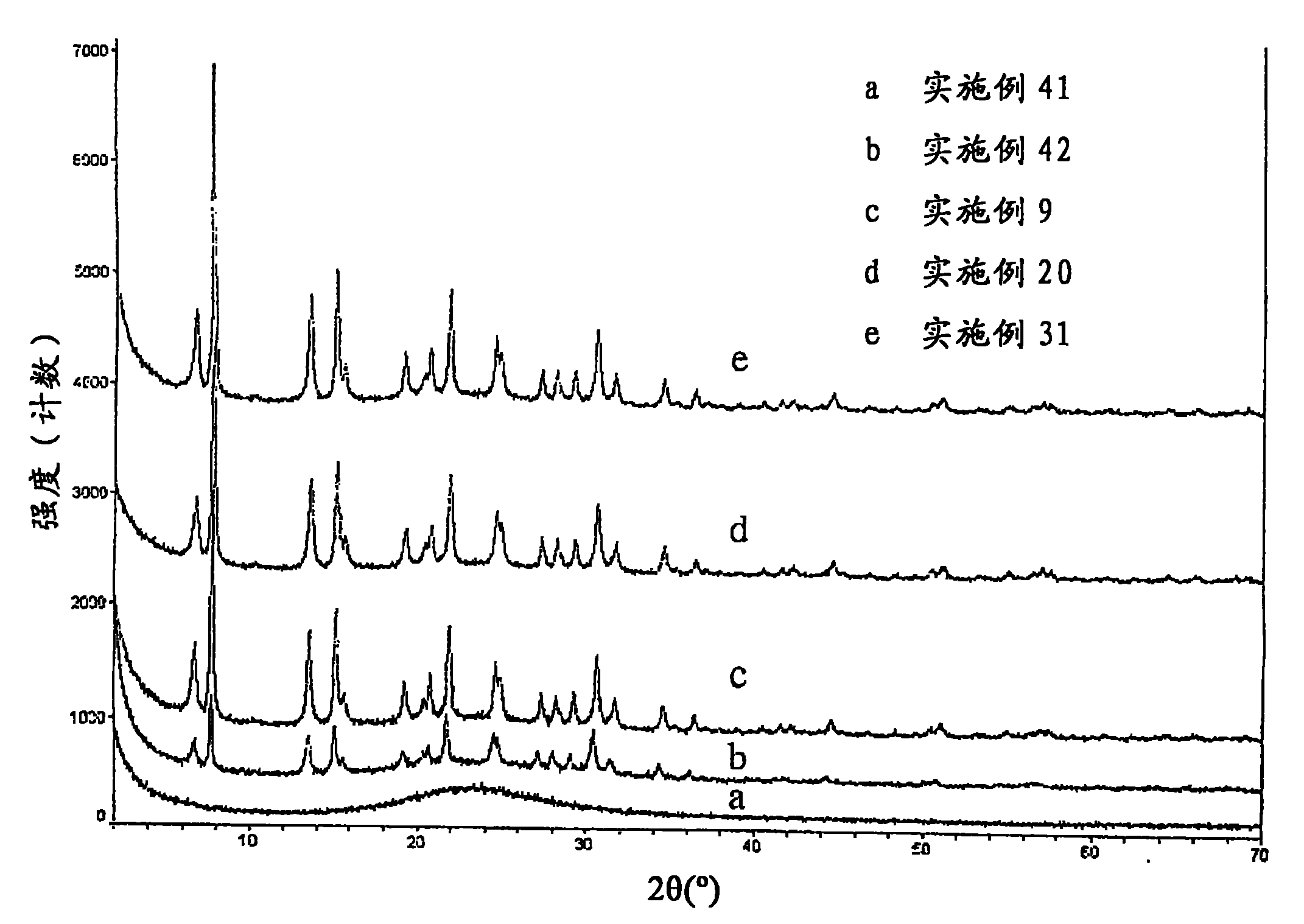
[0098] In a beaker, 6.0 grams (on a fired basis) of NH from Example 2 4 UZM-4 was slurried in 37.8 grams of 3.4M ammonium acetate. The slurry was stirred and heated to 85°C, and a solution containing 1.6 g (NH 4 ) 2 SiF 6 (AFS) solution. After the addition of the AFS solution was complete, the slurry was stirred at 85°C for an additional hour, filtered while hot and the product was washed with warm (50°C) deionized water. The product was then slurried in warm (50°C) deionized water and filtered. Repeat this process two more times. The filtered product was dried at 85°C for 16 hours, then hydrated at ambient conditions and designated UZM-4M (2.7). A comparison of the X-ray powder diffraction patterns of the starting material (UZM-4) and the product UZM-4M (2.7) is shown in Table 1. The observed data are consistent with the retention of crystallinity, indicating that the shrinkage in the unit cell is consistent with the substitution of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com