Mass spectrometric screening of catalysts
A technology of catalyst and catalytic reaction, which is used in mass spectrometer, library screening, chemical analysis using catalysis, etc., and can solve problems such as being unsuitable for high-throughput screening.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Embodiment 1
[0048] Examples Example 1: Screen a small group of Brookhart Pd(II) complexes, these complexes have different aryl groups on the diimine ligand 1aR=CH 3 ; R′=H; R″=H1bR=CH 3 : R′=CH 3 ; R″=H1cR=CH 3 ; R′=CH(CH 3 ) 2 ; R″=H
[0049] The screening process was carried out in accordance with the reaction in Table 1. The solution is composed of complexes 1a, 1b and 1c (see Table 1), where each substance is in CH 2 Cl 2 Is approximately 10 -3 M, the solution was saturated with ethylene and reacted at -30°C for 1 hour, then quenched with dimethyl sulfoxide (DMSO), and finally diluted 100 times. The solution was immediately electrosprayed in the Finnigan MAT TSQ-7000 tandem mass spectrometer, and the operation of the mass spectrometer was carried out as described previously (Hinderling, C. et al. (1997) Angew. Chem. 109: 272; Hinderling, C .et al. (1997) J. Am. Chem. Soc. 119: 10793; Feichtinger, D. and Plattner, DA (1997) Angew. Chem. 109: 1796; Feichtinger, D. et al...
Embodiment 2
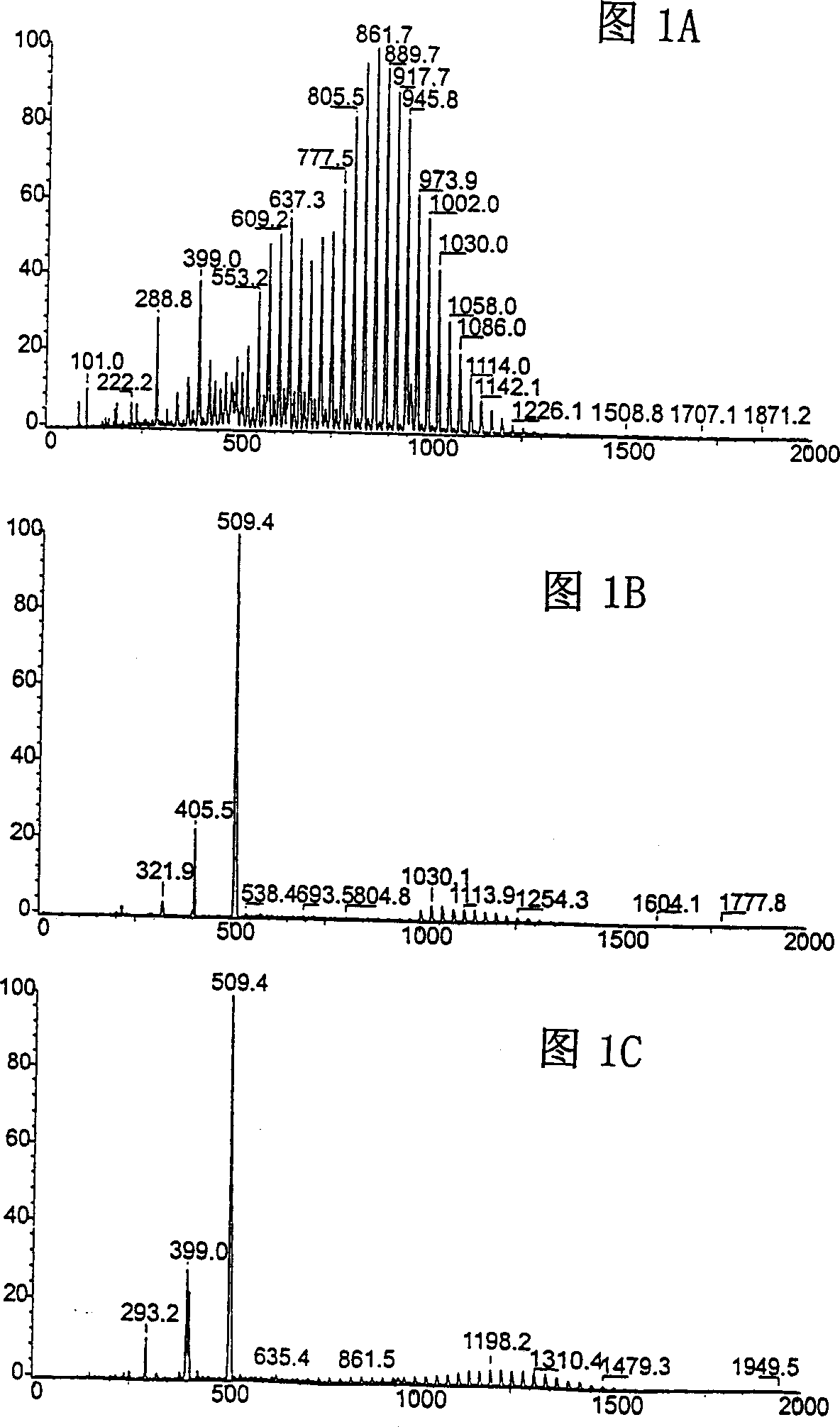
[0049] The screening process was carried out in accordance with the reaction in Table 1. The solution is composed of complexes 1a, 1b and 1c (see Table 1), where each substance is in CH 2 Cl 2 Is approximately 10 -3 M, the solution was saturated with ethylene and reacted at -30°C for 1 hour, then quenched with dimethyl sulfoxide (DMSO), and finally diluted 100 times. The solution was immediately electrosprayed in the Finnigan MAT TSQ-7000 tandem mass spectrometer, and the operation of the mass spectrometer was carried out as described previously (Hinderling, C. et al. (1997) Angew. Chem. 109: 272; Hinderling, C .et al. (1997) J. Am. Chem. Soc. 119: 10793; Feichtinger, D. and Plattner, DA (1997) Angew. Chem. 109: 1796; Feichtinger, D. et al.. (1998) J Am. Chem. Soc. 120: 7175; Hinderling, C. et al. (1998) Angew. Chem. 110: 2831). The electrospray mass spectrum recorded by scanning the first quadrupole in Figure 1A is complex and shows a series of oligomer and polymer ions correspon...
Embodiment 3
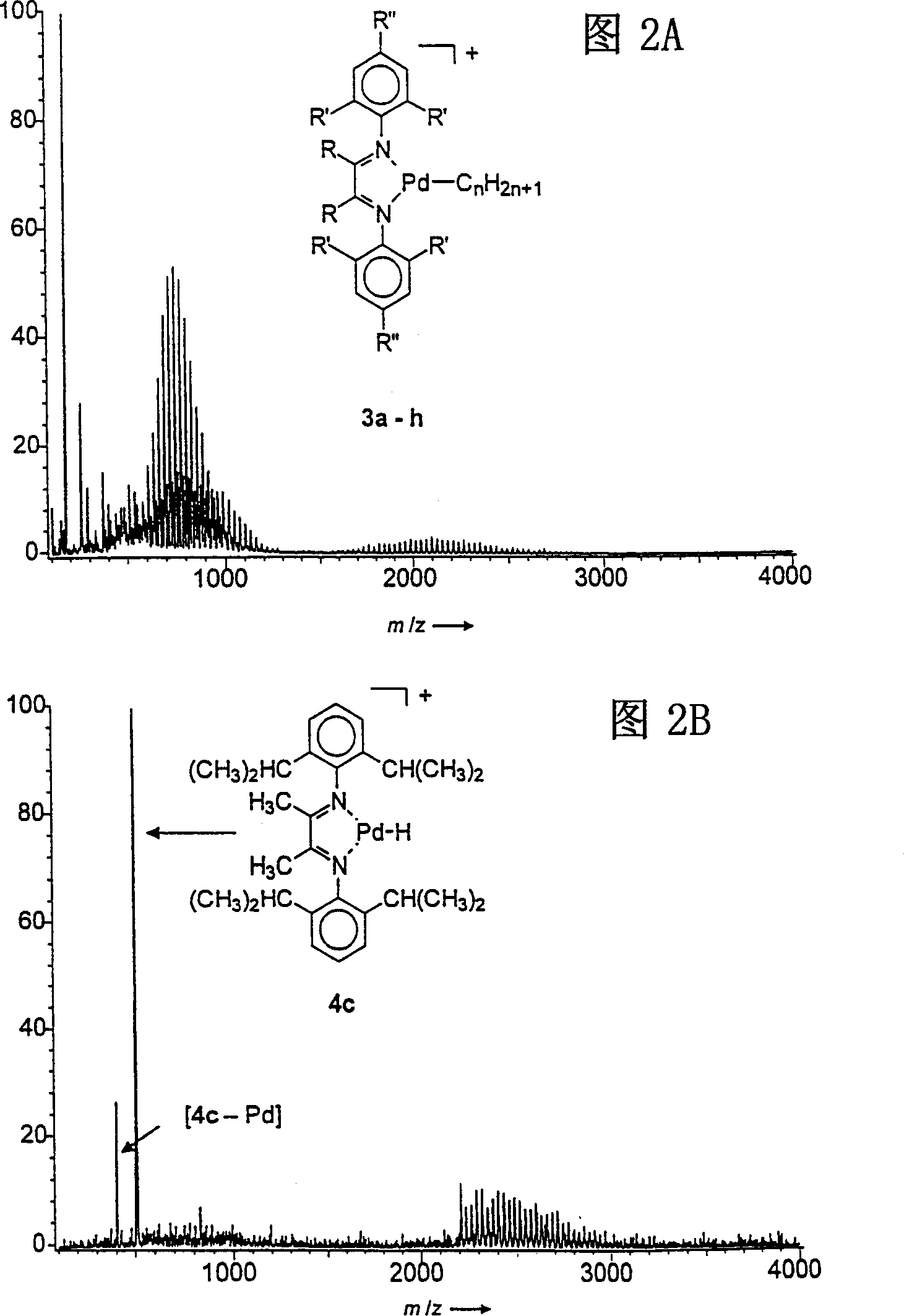
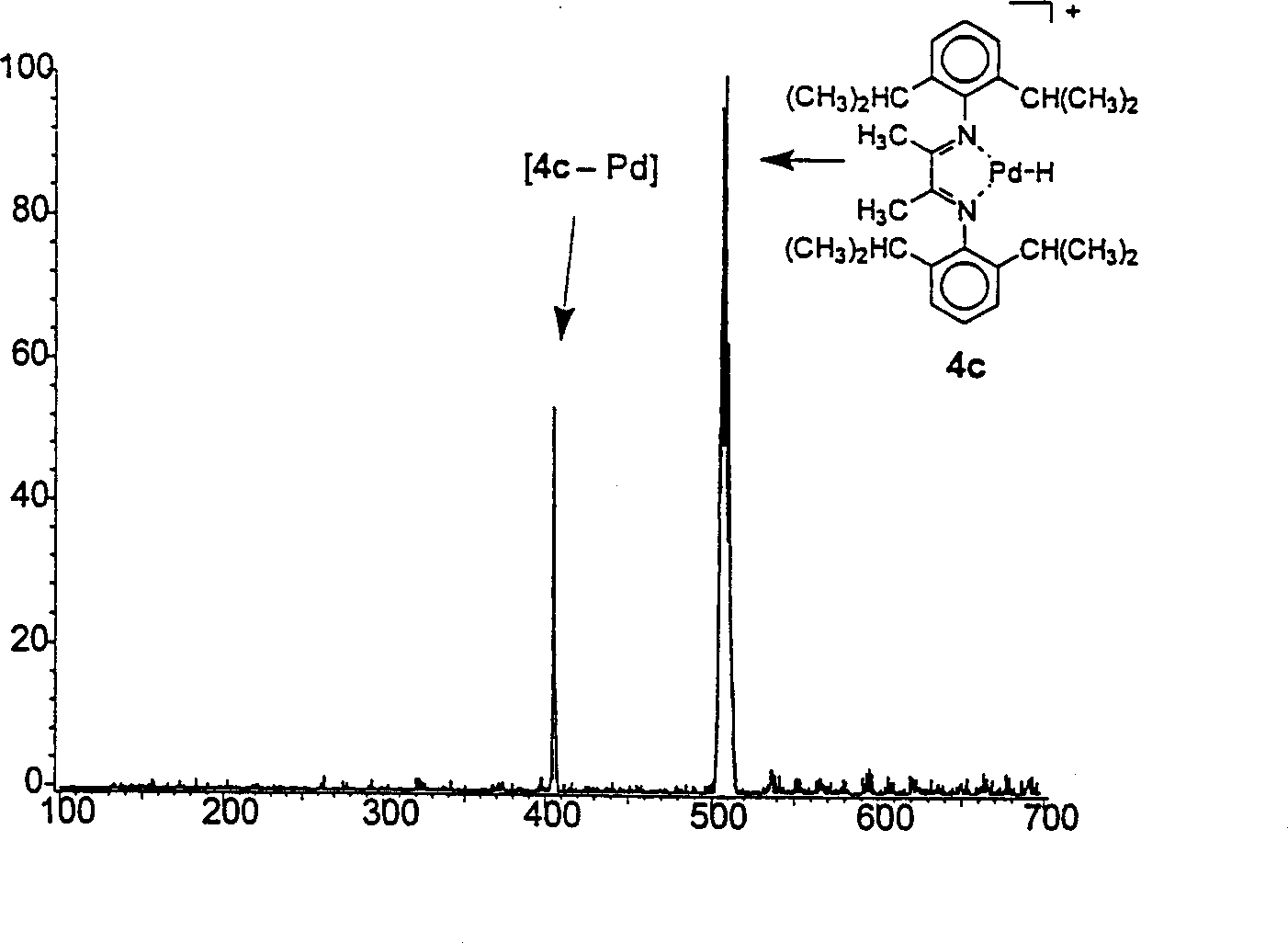
[0053] in Figure 3A It clearly shows that corresponding to ion 4c, the mass peak at m / z=511 is dominant, and ion 4c is formed by collision-induced β-hydride removal of the hydrocarbon chain on the ion formed by catalyst 1c. The small "sharp" peak at m / z=405 corresponds to the secondary fragmentation product of the diimine ligand without palladium. By scanning the precursor ion on the mass of 4 and the secondary fragment, it can be seen that the secondary fragment [4-Pd] (or [4e-Pd-Br]) is clearly connected to the original ion 4. This result clearly shows that among the eight possible catalysts 1a-h, complex 1c is the best actual polymerization catalyst, which is inconsistent with previous reports. [1] Is consistent. Similarly, the product ion mass spectrum with lower mass cutoff mass (Figure 3B) shows that the second best catalysts are 1b, 1d, and 1e. The consistency of mass spectrometry and traditional detection methods proves the effectiveness of the catalyst screening method of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com