High molecular electrolyte and lithium cell
A technology of polymer electrolyte and lithium battery, applied in the field of lithium battery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 4 g of polyethylene glycol having a molecular weight of 400 and 4.205 g of hexamethylene diisocyanate were reacted at 65° C. to prepare a prepolymer having a polyethylene oxide main chain and NCO end caps. Here, 0.092 g (approximately 1% by weight) of dibutyltin dilaurate was used as catalyst.
[0051] Subsequently, 0.085 g of prepolymer was mixed with 0.077 g of glycerol ethoxylate as cross-linking agent, 2.92 g of 1.3M LiPF 6 Mixed with a mixed solution of ethyl carbonate / propylene carbonate / diethyl carbonate at a mixing ratio of 41:49:10 and 0.0235 g of dibutyltin dilaurate. 3 g of the mixture was injected into a battery case with a roll of jelly roll, sealed, and then left to stand for two days. Then, the obtained product was thermally crosslinked at 65° C. for 4 hours to prepare a polymer electrolyte.
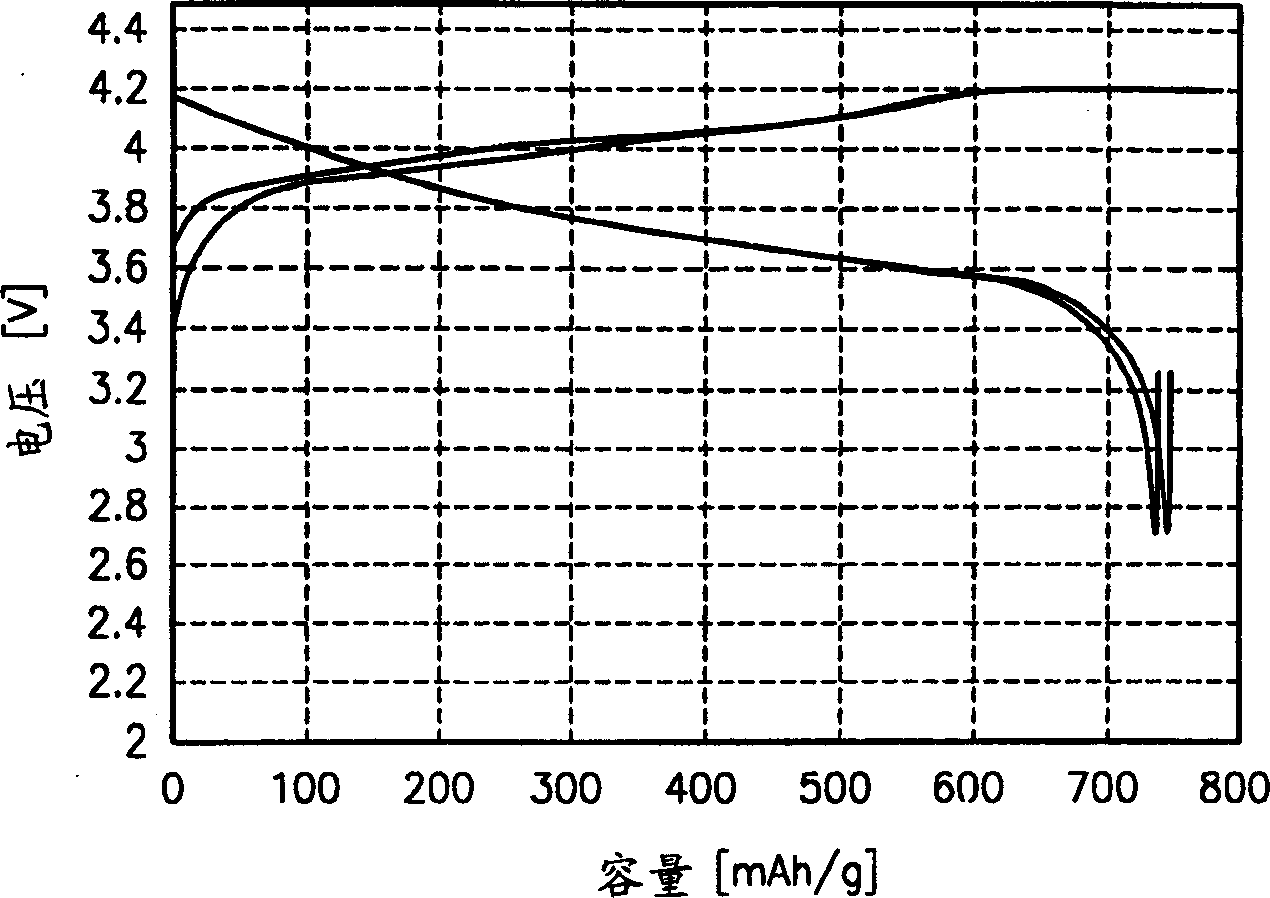
[0052] The standard charge / discharge data (0.5C charge, 0.2C discharge) of a lithium secondary battery (nominal capacity: 800mAh) fabricated using the obtained po...
Embodiment 2
[0054] A prepolymer for forming a polyether amino polymer was prepared in the same manner as in Example 1.
[0055] Subsequently, 0.1 g of the prepolymer was mixed with 0.091 g of glycerol ethoxylate as a crosslinker and 2.28 g of 1.3M LiPF 6 Mixed with a mixed solution of ethyl carbonate / propylene carbonate / diethyl carbonate in a mixing ratio of 41:49:10. The mixture was left at 25° C. for 12 hours to prepare a polymer electrolyte.
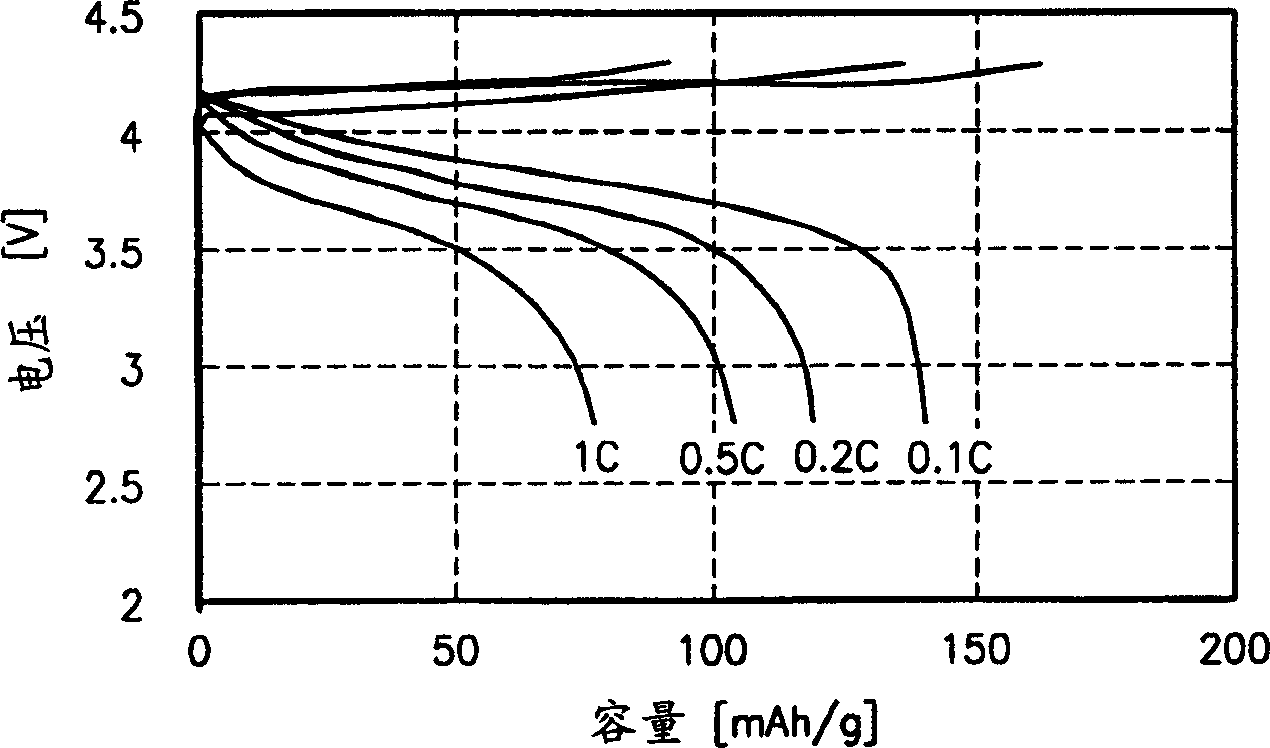
[0056] The polymer electrolyte is placed on the positive electrode (Li) and the negative electrode (LiCoO 2 ) to form a button cell. The charge / discharge characteristics of the button cell are measured by sweeping at 2.7-4.3V, and the results are shown in image 3 middle. Experimental Example 1
experiment Embodiment 1
[0057] This experiment is to measure the electrochemical stability of the polyether urethane polymer electrolyte prepared in Examples 1 and 2.
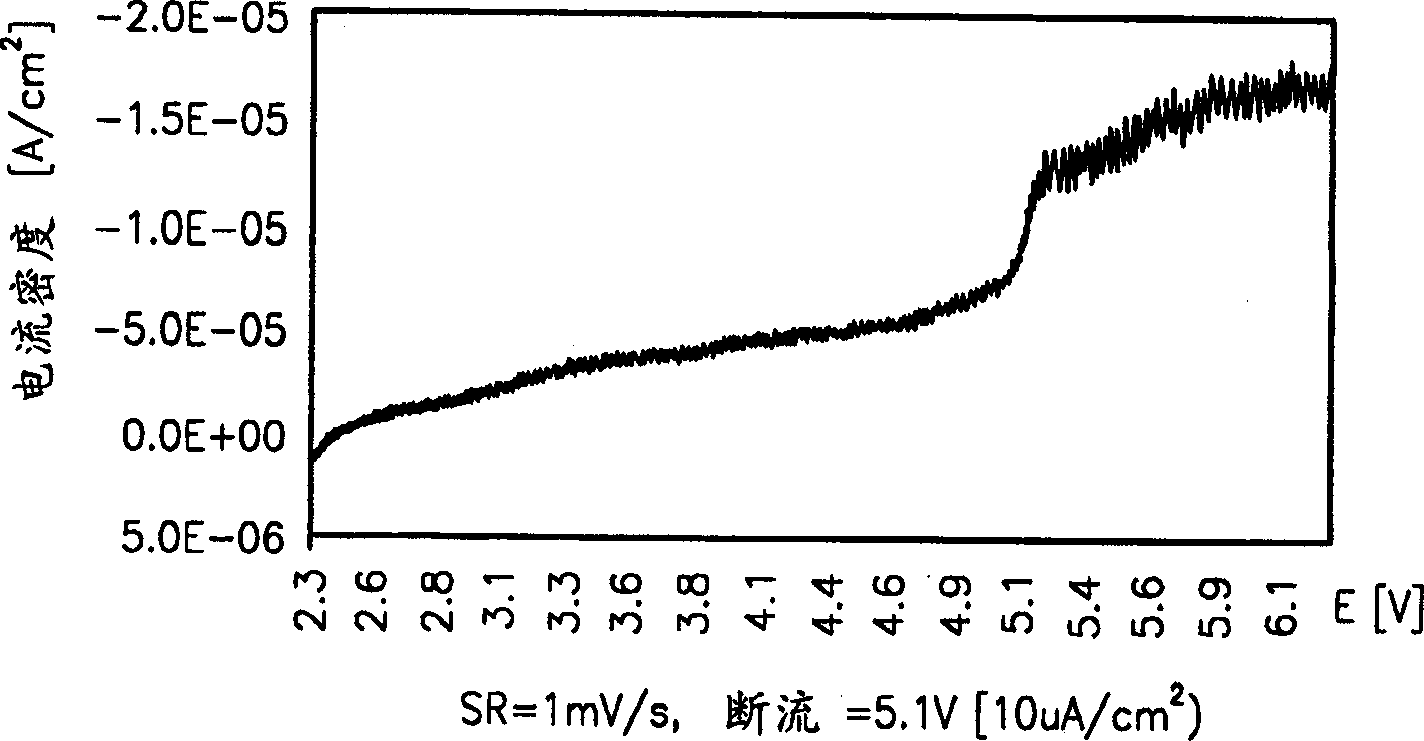
[0058] The dissolution potential of the polyether urethane polymer electrolyte prepared in embodiment 1 is measured with a lithium electrode and a stainless steel (sus) electrode, and the results are shown in figure 1 middle.
[0059] figure 1 Represents the linear purge voltammogram to measure the electrochemical stability of the polymer electrolyte prepared by the present invention, figure 1 It shows that the polyether urethane polymer electrolyte of the present invention is electrochemically stable even at 5.0V or higher.
[0060] Therefore, the polymer electrolyte of the present invention is suitable for lithium secondary batteries, and it must use a polymer electrolyte that is not dangerous when dissolved at 2.75-4.3V.
[0061] Because the lithium secondary battery of the present invention uses an electrochemically stable poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com