Process for preparing rare-earth nano oxide by ball grinding and solid-phase chemical reaction
A solid-phase chemical reaction, nano-rare earth technology, applied in rare earth metal compounds, chemical instruments and methods, inorganic chemistry, etc. The effect of increasing the surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
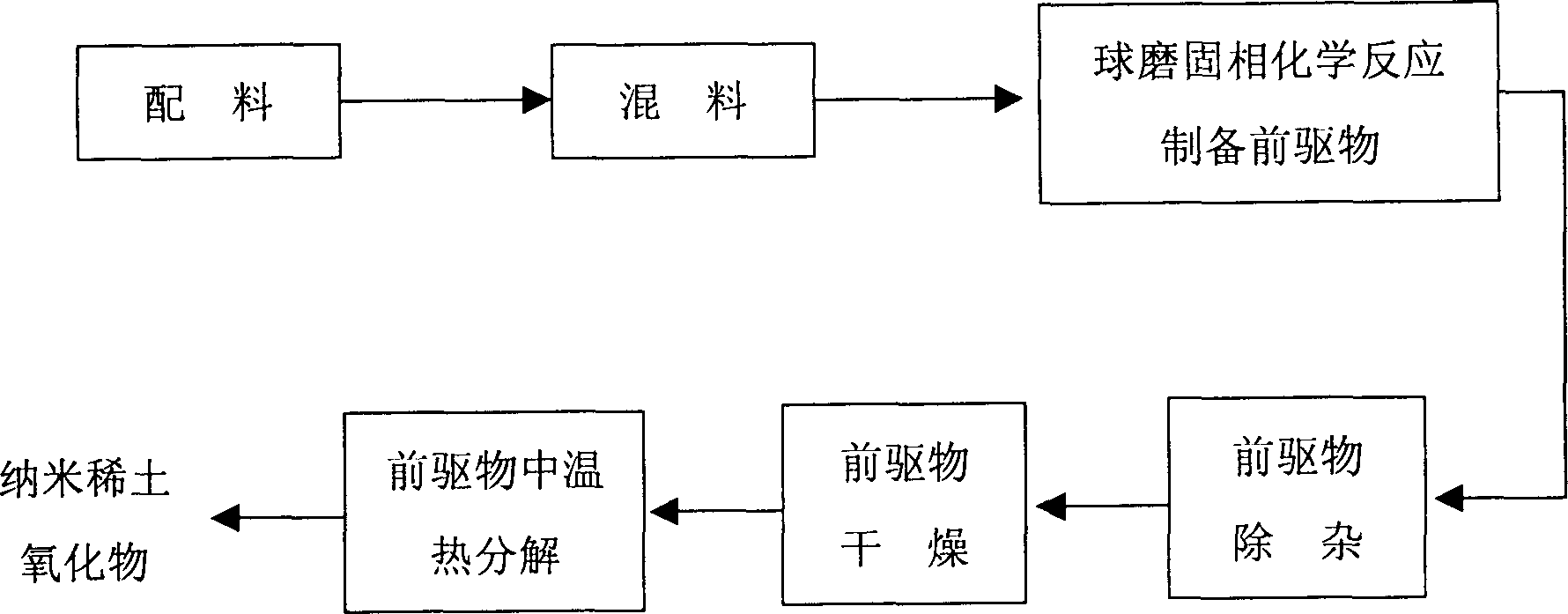
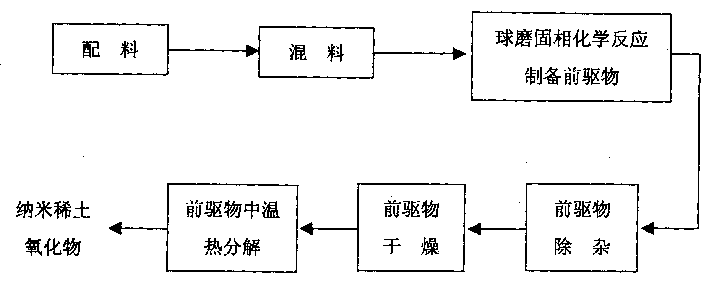
[0029] In this embodiment, the rare earth inorganic salt is cerium nitrate, and the ligand is sodium oxalate. The process flow is as follows: figure 1 As shown, there are the following steps in sequence:
[0030] (1) Ingredients
[0031] The molar ratio of cerium nitrate and sodium oxalate is 1: 4;
[0032] (2) Mixing
[0033] Mix cerium nitrate and sodium oxalate until uniform;
[0034] (3) Preparation of precursors by ball milling solid-phase chemical reaction
[0035] Place the mixture of cerium nitrate and sodium oxalate in a star-shaped ball mill and add lubricant ethanol for ball milling. The amount of ethanol added is limited to keep the dispersed state of the ball mill. The speed of the ball mill is controlled at 230 rpm. The ball mill is at room temperature, Carried out under normal pressure, cerium nitrate and sodium oxalate can be completely chemically reacted to form the precursor cerium oxalate in 20 minutes;
[0036] (4) Precursor removal
[0037] Cerium ox...
Embodiment 2
[0043] In this embodiment, the rare earth inorganic salt is cerium ammonium nitrate, and the ligand is oxalic acid. The process flow is as follows: figure 1 As shown, there are the following steps in sequence:
[0044] (1) Ingredients
[0045] The molar ratio of ceric ammonium nitrate and oxalic acid is 1: 4;
[0046] (2) Mixing
[0047] Mix ammonium cerium nitrate and oxalic acid until uniform;
[0048] (3) Preparation of precursors by ball milling solid-phase chemical reaction
[0049] The mixture of ammonium cerium nitrate and oxalic acid is placed in a star-shaped ball mill and the lubricant ethanol is added for ball milling. The amount of ethanol added is limited to keep the dispersed state of the ball mill, the speed of the ball mill is controlled at 270 rpm, and the ball mill is at room temperature, Carried out under normal pressure, cerium ammonium nitrate and oxalic acid can be completely chemically reacted to form the precursor cerium oxalate in 15 minutes;
[0...
Embodiment 3
[0057] In this embodiment, the rare earth inorganic salt is cerium chloride, and the ligand is sodium bicarbonate, and the process flow is as follows figure 1 As shown, there are the following steps in sequence:
[0058] (1) Ingredients
[0059] The mol ratio of cerium chloride and sodium bicarbonate is 1: 8;
[0060] (2) Mixing
[0061] Mix cerium chloride and sodium bicarbonate until uniform;
[0062] (3) Preparation of precursors by ball milling solid-phase chemical reaction
[0063] The mixture of cerium chloride and sodium bicarbonate is placed in a star-shaped ball mill and added lubricant ethanol for ball milling. The amount of ethanol added is limited to keep the dispersed state of the ball mill. The speed of the ball mill is controlled at 250 rpm. Carried out at room temperature and normal pressure, the chemical reaction between cerium chloride and sodium bicarbonate can completely occur in 25 minutes to form the precursor cerium bicarbonate;
[0064] (4) Precurs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com