Desmocyte growth factor 11 antibody, antagonist and agonist
A cell and antibody technology, applied in biological testing, anti-fungal/algae/lichen immunoglobulin, material inspection products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Example 1 Bacterial expression and purification of FGF-11 protein
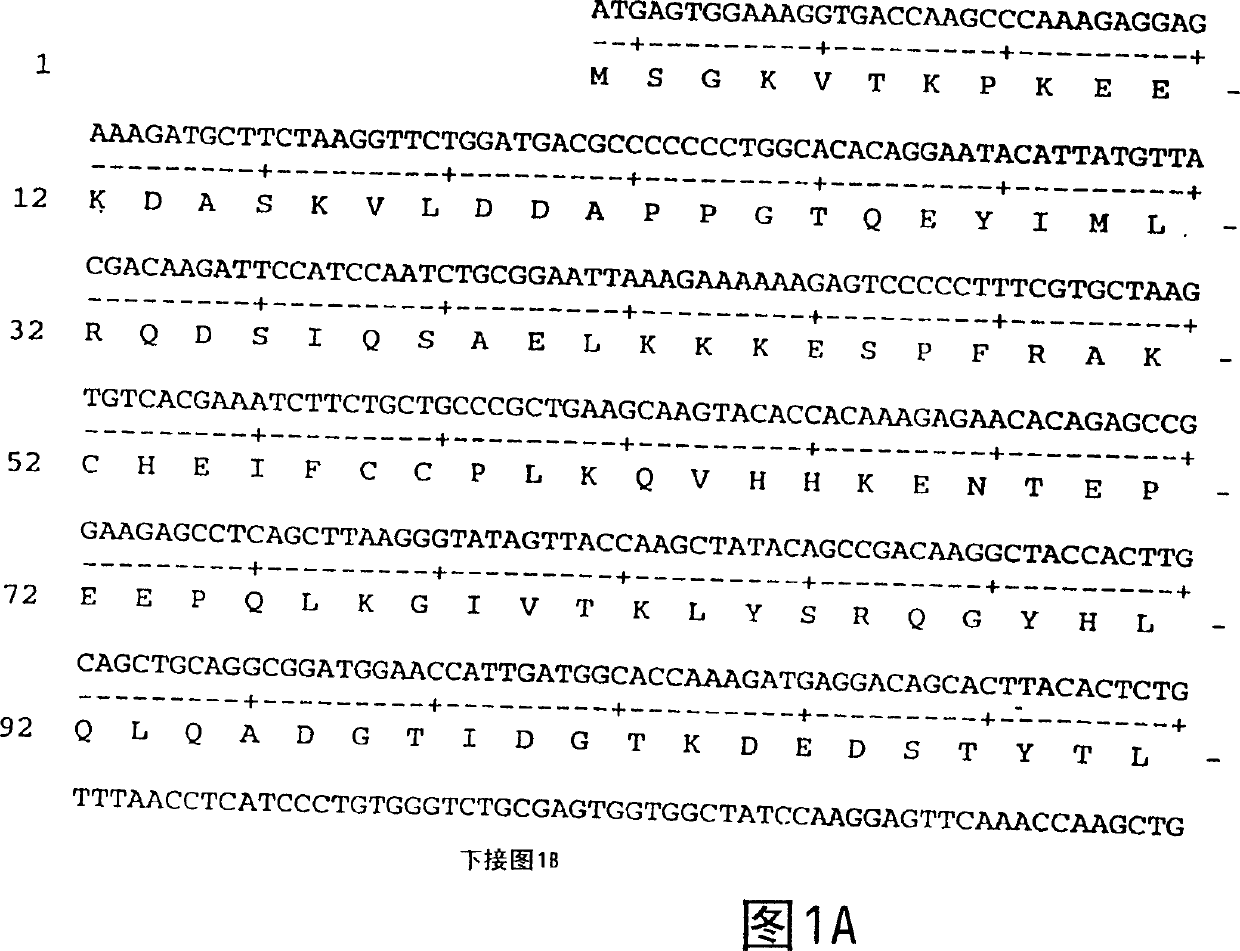
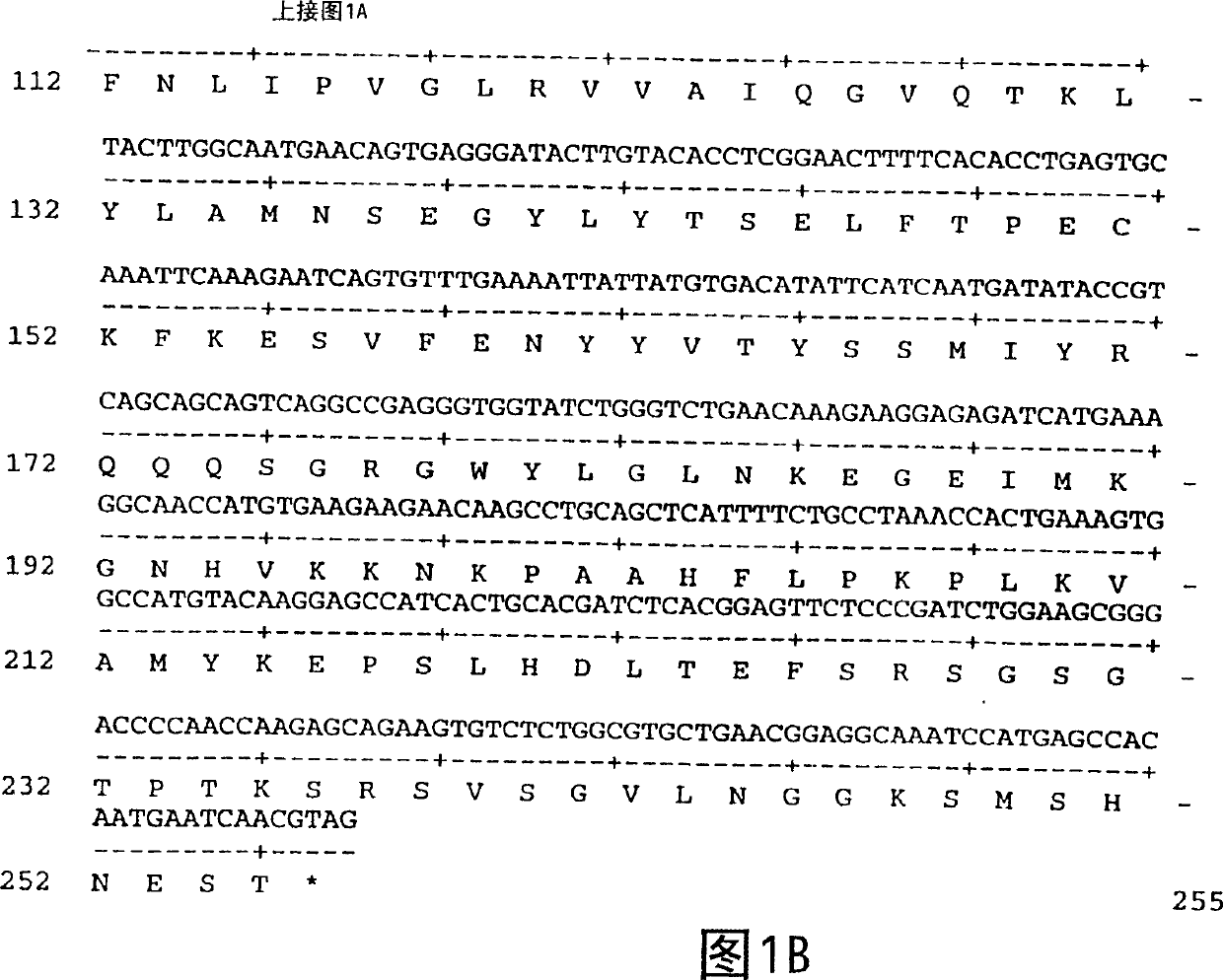
[0139] Amplification of the DNA sequence encoding FGF-11 was initiated using PCR oligonucleotide primers, ATCC #97150, which correspond to the 5' sequence of the processed FGF-11 protein (minus the signal peptide sequence) and the FGF-11 Vector sequences 3' of the 11 genes. Additional nucleotides corresponding to the FGF-11 gene were added to the 5' and 3' sequences, respectively. The 5' oligonucleotide primer 5'CGCGGATCCATCATGAGTGGAAAGGTGACCAAG 3' (SEQ ID NO: 3) contains a BamHI restriction endonuclease site. The 3' sequence 5'CGCGGATCCCGTTGATTCATTGTGGCTCAT 3' (SEQ ID NO: 4) contains the sequence complementary to the BamHI site followed by 21 nucleotides of the FGF-11 coding sequence.
[0140] The restriction enzyme sites correspond to the restriction enzyme sites of the bacterial expression vector pQE-60 (Qiagen, Chatsworth Corporation, CA, 91311). pQE-60 encodes antibiotic resistance (Amp r ), ba...
Embodiment 2
[0140] The restriction enzyme sites correspond to the restriction enzyme sites of the bacterial expression vector pQE-60 (Qiagen, Chatsworth Corporation, CA, 91311). pQE-60 encodes antibiotic resistance (Amp r ), bacterial origin of replication (ori), IPTG regulatable promoter operator (P / O), ribosome binding site (RBS), 6-histidine tag and restriction endonuclease sites. pQE-60 was then digested with NcoI and BamHI. The amplified sequence was ligated into pQE-60 and inserted in frame with the histidine tag coding sequence and ribosome binding site (RBS). The ligation mixture was then used to transform E. coli strain M15 / rep4 (Qiagen, Inc.) by the method described by Sambrook et al. (Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, (1989)). M15 / rep4 contains multiple copies of the plasmid pREP4, which expresses the lacI repressor and confers kanamycin resistance (Kan r ). Transformants were identified by their ability to grow on LB plates, and a...
Embodiment 3
[0143] Example 3 Cloning and expression of FGF-11 using the baculovirus expression system
[0144] The DNA sequence encoding the full-length FGF-11 protein (ATCC #97150) was amplified using PCR oligonucleotide primers corresponding to the 5' and 3' sequences of the gene:
[0145] The FGF-11 5' primer has the sequence 5'CGCGGATCCATCATGAGTGGAAAGGTGACCAAG 3' (SEQ ID NO: 5) and contains a BamHI restriction site (bold) so that cloning at this site places the baculovirus signal sequence in the putative FGF -11 signal peptide cleavage site downstream of the FGF-11 gene 21 nucleotide reading frame.
[0146] The 3' primer has the sequence 5'CGCGGTACCCTACGTTGATTCATTGTGGCT 3' (SEQ ID NO: 6), contains a cleavage site for restriction endonuclease Asp718 and 21 nucleotides complementary to the 3'-untranslated sequence of the gene.
[0147] The amplified sequence was separated from a 1% agarose gel using a commercially available kit ("Geneclean" BIO 101 Company, La Jolla, Ca.). The fragmen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com