Slow-released indapamide capsule and its prepn process
A technology for indapamide and sustained-release capsules, which is applied in the field of indapamide sustained-release capsules and its preparation, can solve the problems of unsuitable popularization, large side effects, and high price, and achieve shortened processing time, complete absorption, and low cost. cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
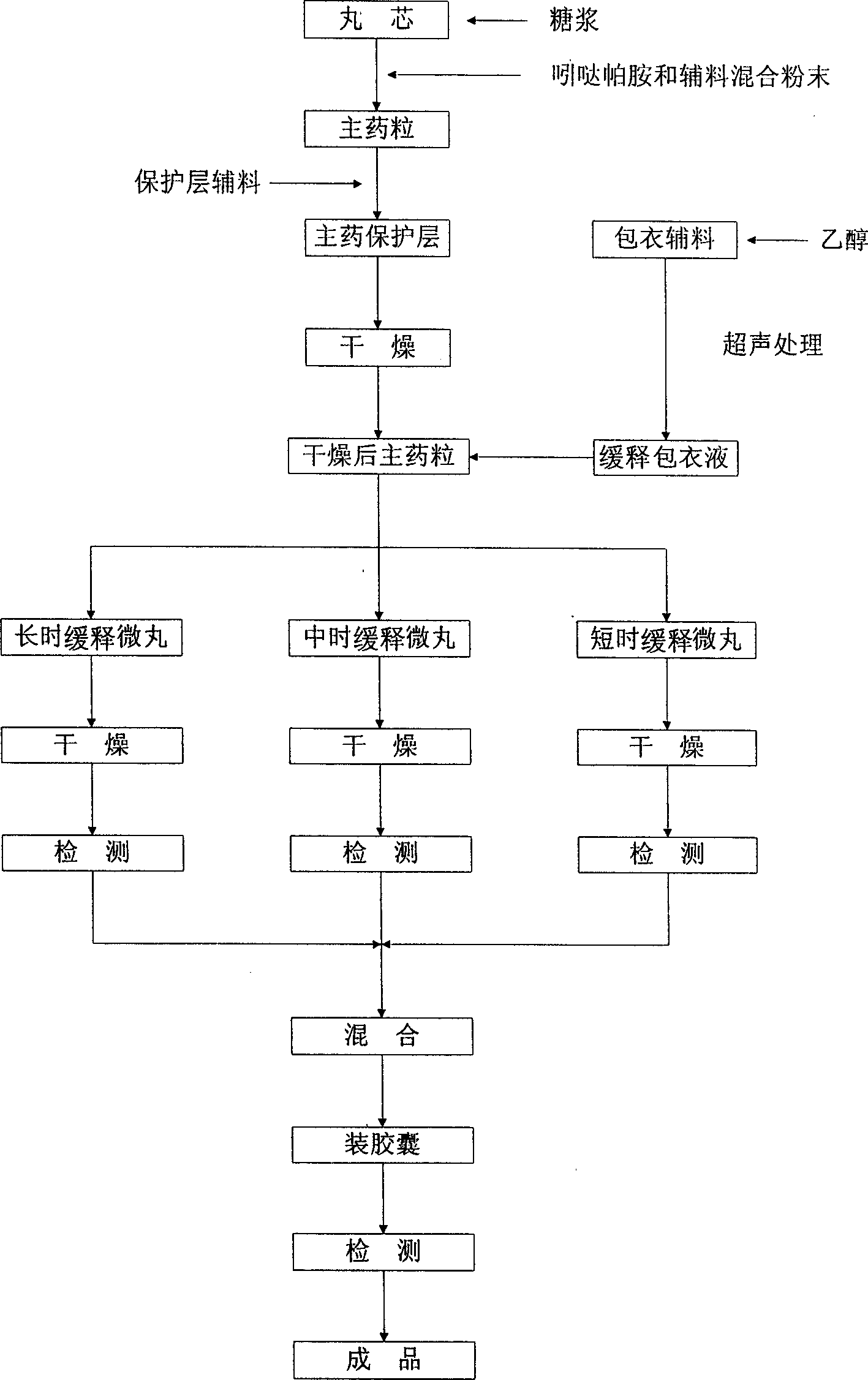
Image
Examples
Embodiment 1
[0013] Based on 1000 indapamide sustained-release capsules, put 105 g of commercially available hollow ball cores between 25 and 30 meshes in a CF300 granulator, and spray 50% sucrose syrup until the hollow ball cores are fully moistened. Wet; mix 1.5g of indapamide through a 100-mesh sieve, 20g of lactose through a 80-mesh sieve, and 20g of starch powder through a 100-mesh sieve, then add it to the above-mentioned granulator and process for 50 minutes to obtain the main ingredient with a smooth surface After the main drug granules are wrapped, continue to spray 50% sucrose syrup, and add 8g of starch to wrap the main drug protective layer, and then dry the wrapped main drug granules in a vacuum oven at 80°C for 3 hours, or Dry in an ordinary electric drying oven at 80°C for 6 hours; dissolve 10g of the acrylic resin sustained-release material EUDRAGIT RS100 with an appropriate amount of 95% ethanol, and add 3g of talcum powder, an auxiliary material for coating, into an approp...
Embodiment 2
[0015] Based on 1000 indapamide sustained-release capsules, put 105 g of commercially available hollow ball cores between 25 and 30 meshes in a CF300 granulator, and spray 50% sucrose syrup until the hollow ball cores are fully moistened. Wet; mix 1.5g of indapamide passing through a 100-mesh sieve, 15g of polyethylene glycol 6000 passing through a 80-mesh sieve, and 13g of pregelatinized starch powder passing through a 100-mesh sieve and add it to the above-mentioned granulator for 50 minutes To obtain the main drug granules with smooth surface, after the main drug granules are wrapped, continue to spray 50% sucrose syrup, and add 5g of starch to wrap the main drug protective layer, and then place the wrapped main drug granules in a vacuum drying at 80°C Dry in an oven for 3 hours, or dry in an ordinary electric drying oven at 80°C for 6 hours; dissolve 8g of polyvinylpyrrolidone with an appropriate amount of 95% ethanol and 12g of hydroxypropyl methylcellulose with 5mL of wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com