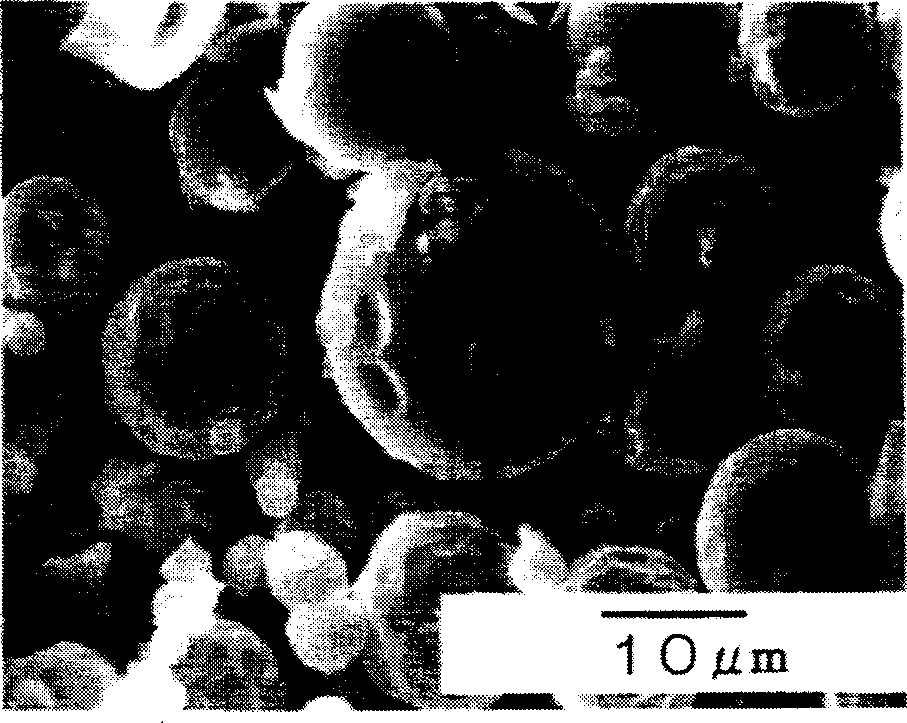Process for producing fluorescent metal oxide material
A manufacturing method and oxide technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problem that metal chelates are not fully utilized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 166.35 g (0.57 mol) of ethylenediaminetetraacetic acid and water were added to a 2-liter beaker so that the total amount would be 700 g, and then 75 g of aqueous ammonia was added and dissolved. The solution was heated while stirring, and while stirring at a solution temperature of 60° C., 115.44 g (yttrium: 0.57×0.98 mol) of yttrium carbonate trihydrate (yttrium content: 43.0%) and europium oxide (europium content: 85.3 mol) were slowly added in sequence. %) 2.03g (europium: 0.57×0.02mol), and keep it from boiling and overflowing. Then, stirring was continued at 100° C. for 3 hours, and the pH became 5.0, and the solution was completely dissolved. Water was added thereto to make the total amount 1500 g, at this time a colorless and clear organic chelate solution with a metal molar ratio of Y:Eu=0.98:0.02 was obtained. The solution was powdered by spray drying at a drying temperature of 160°C to obtain a mixed metal complex powder (Y 2 o 3 : Eu) 137g.
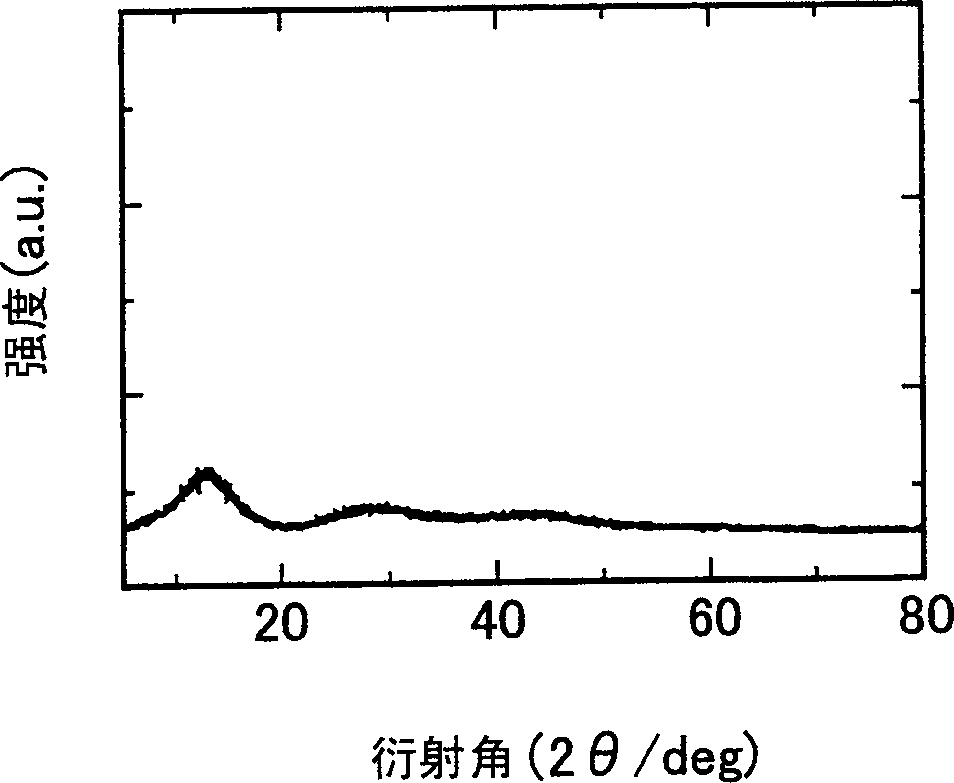
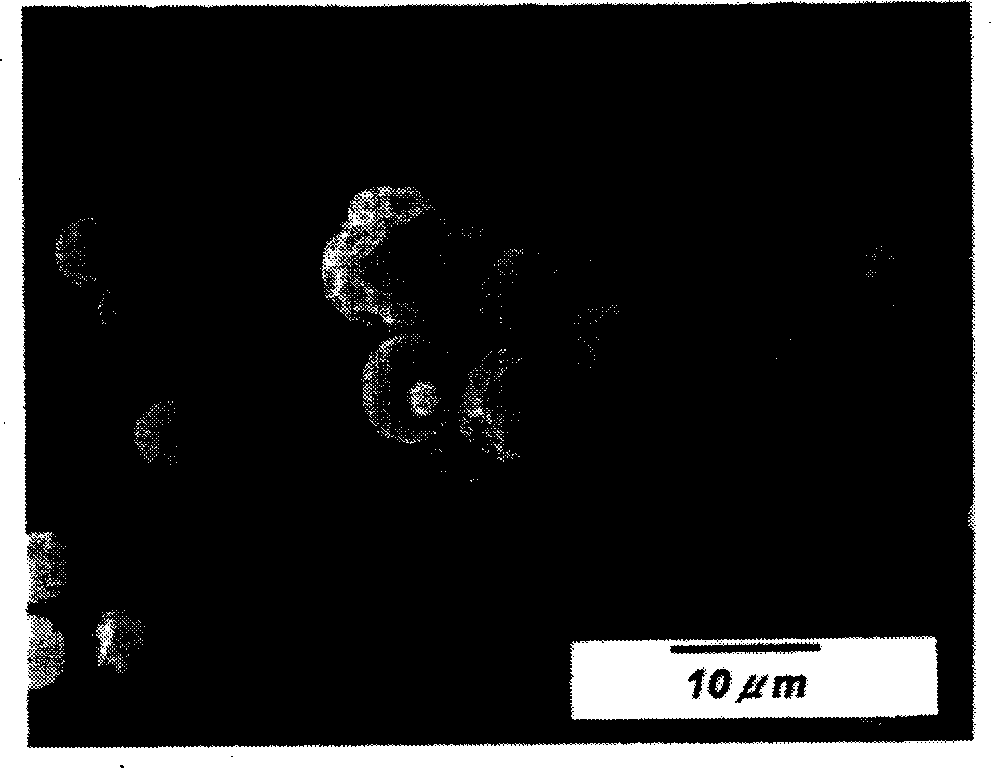
[0050] figu...
Embodiment 2
[0056] After adding 21.58 g (0.074 mol) of ethylenediaminetetraacetic acid and water to a 1-liter beaker so that the total amount would be 500 g, 10 g of ammonia water was added and dissolved. The solution was heated while stirring, and while stirring at a solution temperature of 60° C., 6.44 g of barium carbonate (barium content: 69.1%) (barium: 0.033 mol), magnesium hydroxide (magnesium content: 41.1%) 2.14 g were slowly added in sequence. g (magnesium: 0.036mol) and europium oxide (europium content: 85.3%) 0.645g (europium: 0.0036mol), and keep it from boiling to overflow. Then, the stirring was continued at 80° C. for 1 hour, and then 6 g of aqueous ammonia was added to dissolve completely. Next, 137.66 g (aluminum: 0.36 mol) of ethylenediaminetetraacetic acid aluminum-ammonium salt (aluminum content: 7.1%) was added to this solution, and the pH became 6.7 and the solution was completely dissolved. Continue to add water in this solution, make total amount become 1000g, at...
Embodiment 3
[0064] 10.02 g (0.034 mol) of ethylenediaminetetraacetic acid and water were added to a 1-liter beaker so that the total amount would be 500 g, and then 5 g of ammonia water was added and dissolved. The solution was heated while stirring, and while stirring at a solution temperature of 60° C., slowly added barium carbonate (barium content: 69.1%) 6.35 g (barium: 0.032 mol) and manganese carbonate (manganese content: 43.5%) 0.213 g (manganese: 0.0017mol), and keep it from boiling and overflowing. Then, the stirring was continued at 80° C. for 1 hour, and 4 g of ammonia water was added to dissolve completely. Add aluminum-ammonium edetate (aluminum content: 7.1%) 153.43g (aluminum: 0.404mol) to this solution, then pH becomes 6.5 and dissolve completely, then continue to add water in this solution, make total amount become 1000g, At this time, a colorless and clear organic chelate solution with metal molar ratio (Ba+Mn):Al=1:12, Ba:Mn=0.95:0.05 can be obtained. The solution was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com