Preparation method of crystal phase controllable titanium dioxide nanometer crystal
A titanium dioxide and nanocrystalline technology, applied in the direction of titanium dioxide, titanium oxide/hydroxide, etc., can solve the problems that the ratio of anatase and rutile cannot be continuously modulated, difficult to promote industrially, and the preparation cost is high, and it is easy to achieve process parameters. Control, easy industrial promotion, short production cycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 10
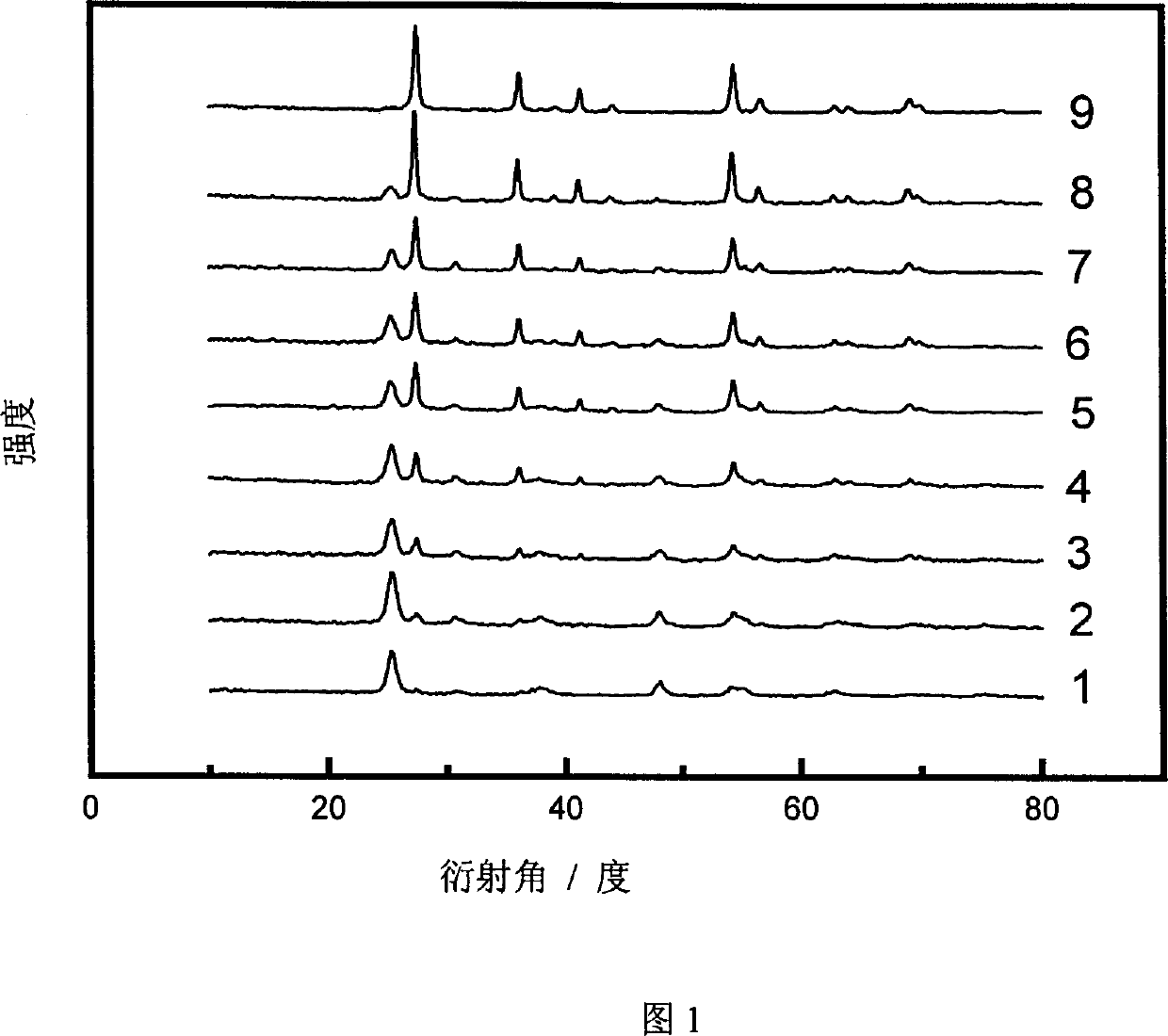
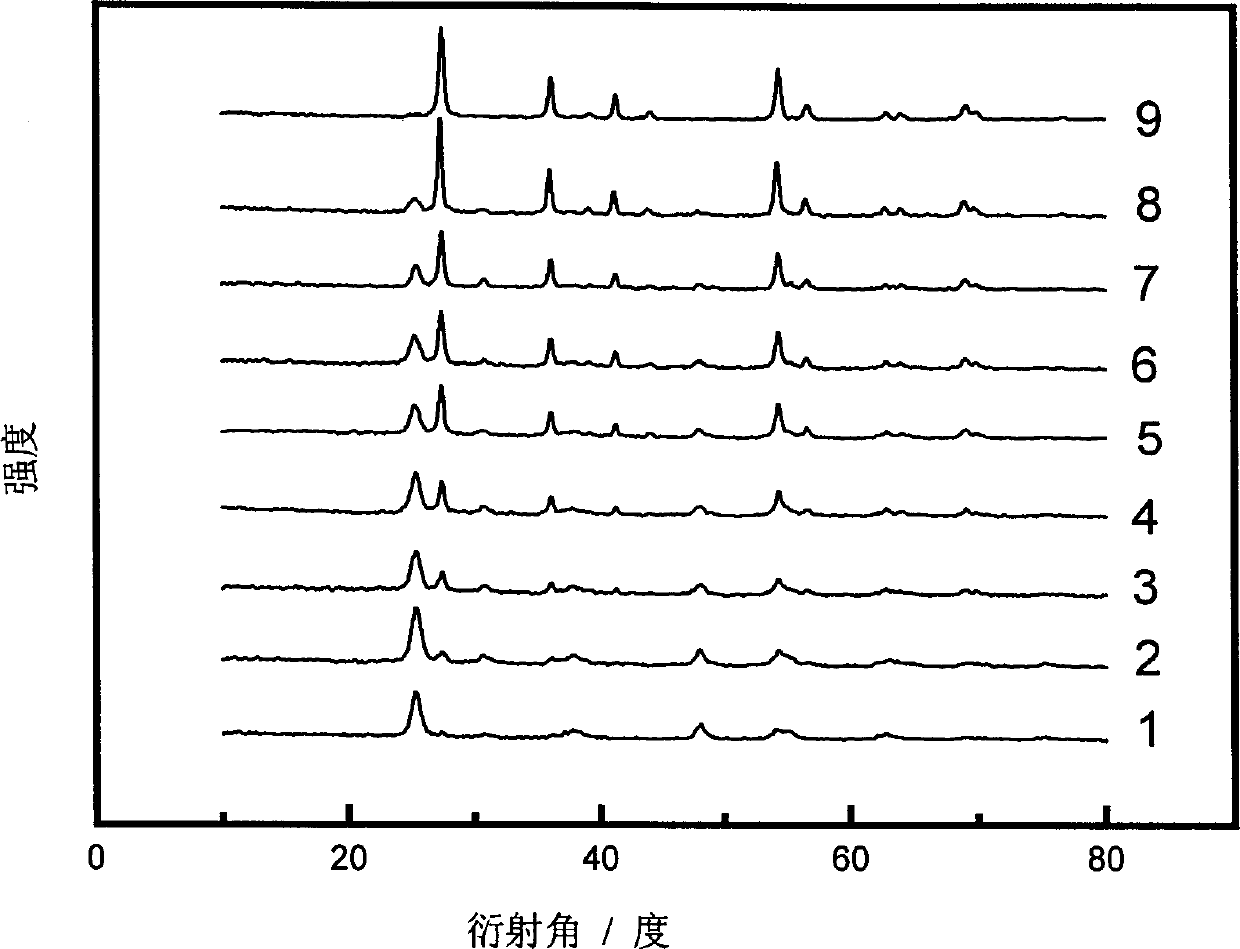
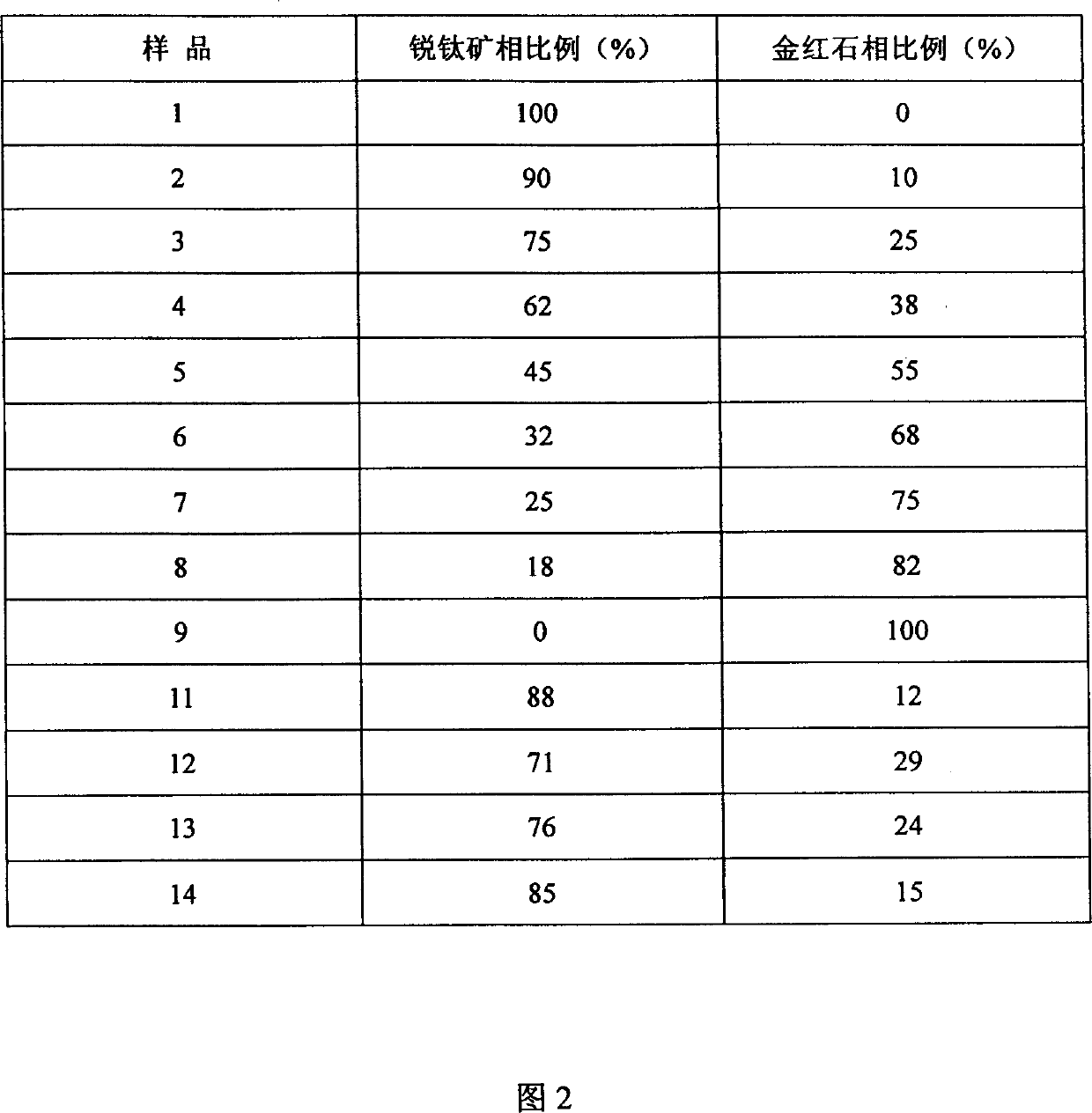
[0025] The XRD patterns of samples 1-9 were measured on a Rigaku D / MAX-IIA diffractometer, and the peaks at 25.2, 38.7 and 48.0° belonged to the diffraction of anatase (101), (004) and (200) crystal planes, respectively , and the peaks at 27.4, 36.1, 41.2, and 54.3° are assigned to the diffraction of rutile (110), (101), (111) and (211) crystal planes, respectively. The proportion of anatase phase and rutile phase in titanium dioxide using the phase quantification formula x A =(1+1.26I R / I A ) -1 Calculate (C.C. Wang, J.Y. Ying, Chem. Mater, 1999; 11:3113), where x A is the proportion of anatase phase, I A and I R are the intensities of the diffraction peaks attributed to anatase and rutile, respectively, and the results are shown in Table 1. Examples 11~12
example 11~12
[0026] Dissolve 0.1 mol of butyl titanate in 1.5 mol of absolute ethanol, add dropwise a mixture of 1 mol of ethanol and 5 mol of water, stir at room temperature for 3 hours, then filter with suction and dry at room temperature to obtain amorphous titanium dioxide powder. Add a certain amount of hydrochloric acid (20mL / g titanium dioxide powder) to the amorphous titanium dioxide powder, the concentration is 0.2, 0.3mol / L respectively, stir at room temperature for 1 hour, 180 ℃ hydrothermal crystallization for 24 hours, after suction filtration, Titanium dioxide samples were obtained by washing and drying, which were respectively recorded as samples 11 and 12, and the proportion of the anatase phase was determined by the method of Example 10. Examples 13-14
example 13~14
[0027] Dissolve 40ml of titanium tetrachloride in 100mL of 0.6N hydrochloric acid, and then add dropwise 1:1 ammonia water until the pH value is 9-10. Aging at room temperature for 12 hours, suction filtration, washing with distilled water until there is no chloride ion, drying and grinding to obtain amorphous titanium dioxide powder. Add a certain amount of hydrochloric acid (10mL / g titanium dioxide powder) to the amorphous titanium dioxide powder, the concentration is 0.3mol / L, stir at room temperature for 1 hour, 150°C hydrothermal crystallization for 24 and 48 hours respectively, after suction filtration, Titanium dioxide samples were obtained by washing and drying, respectively recorded as samples 13 and 14, and the proportion of the anatase phase was determined by the method of Example 10. Examples 15-18
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com