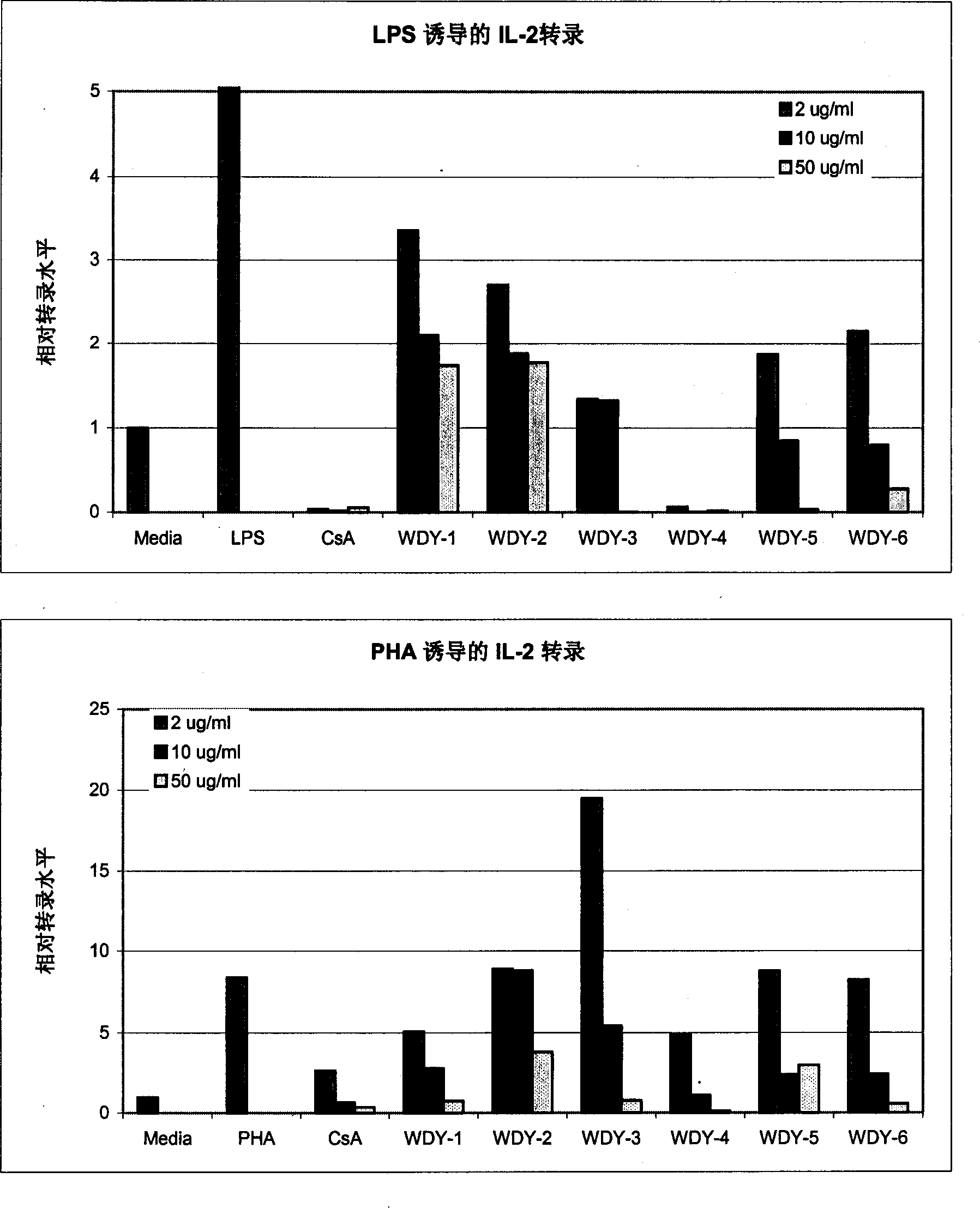
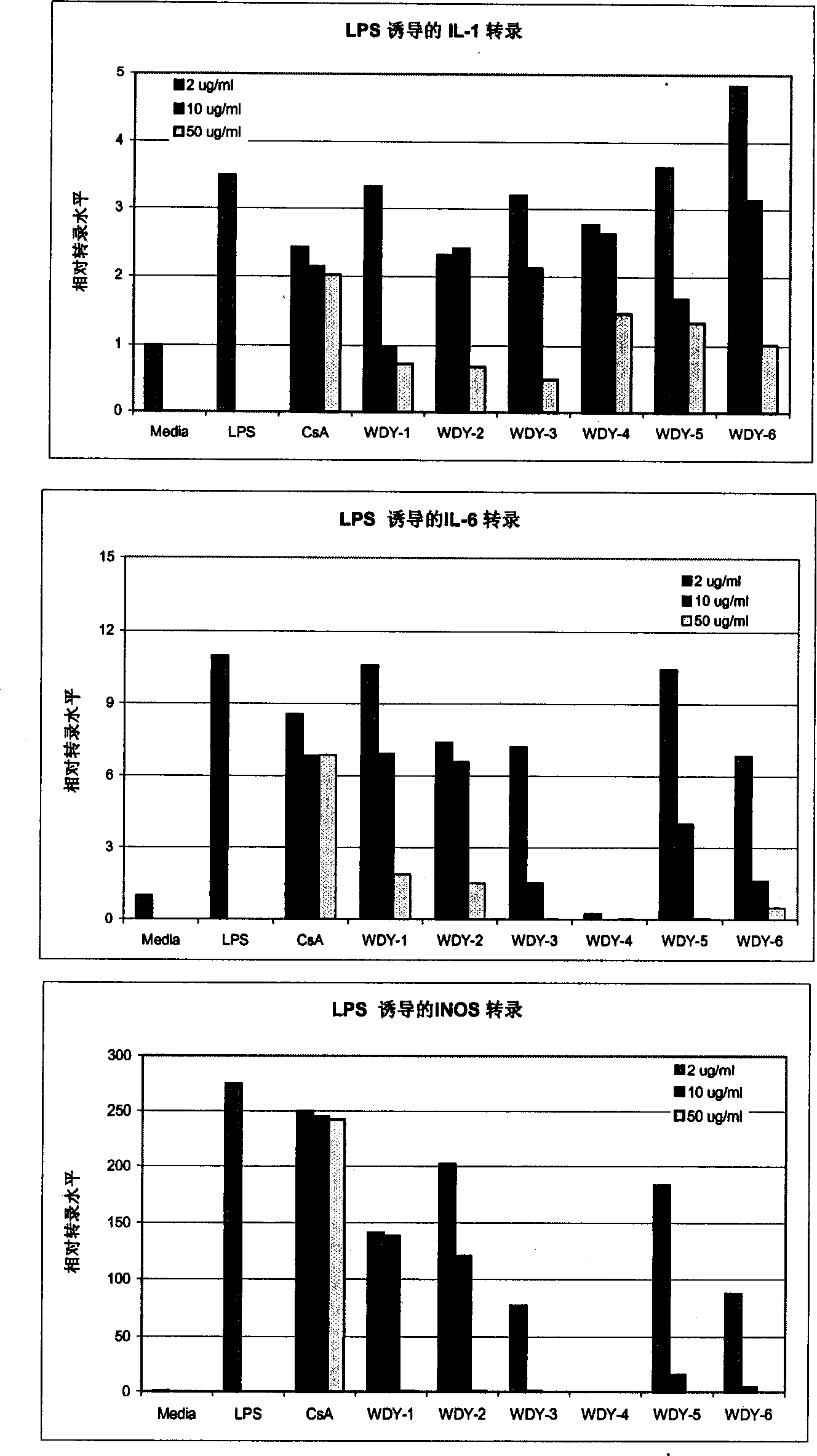
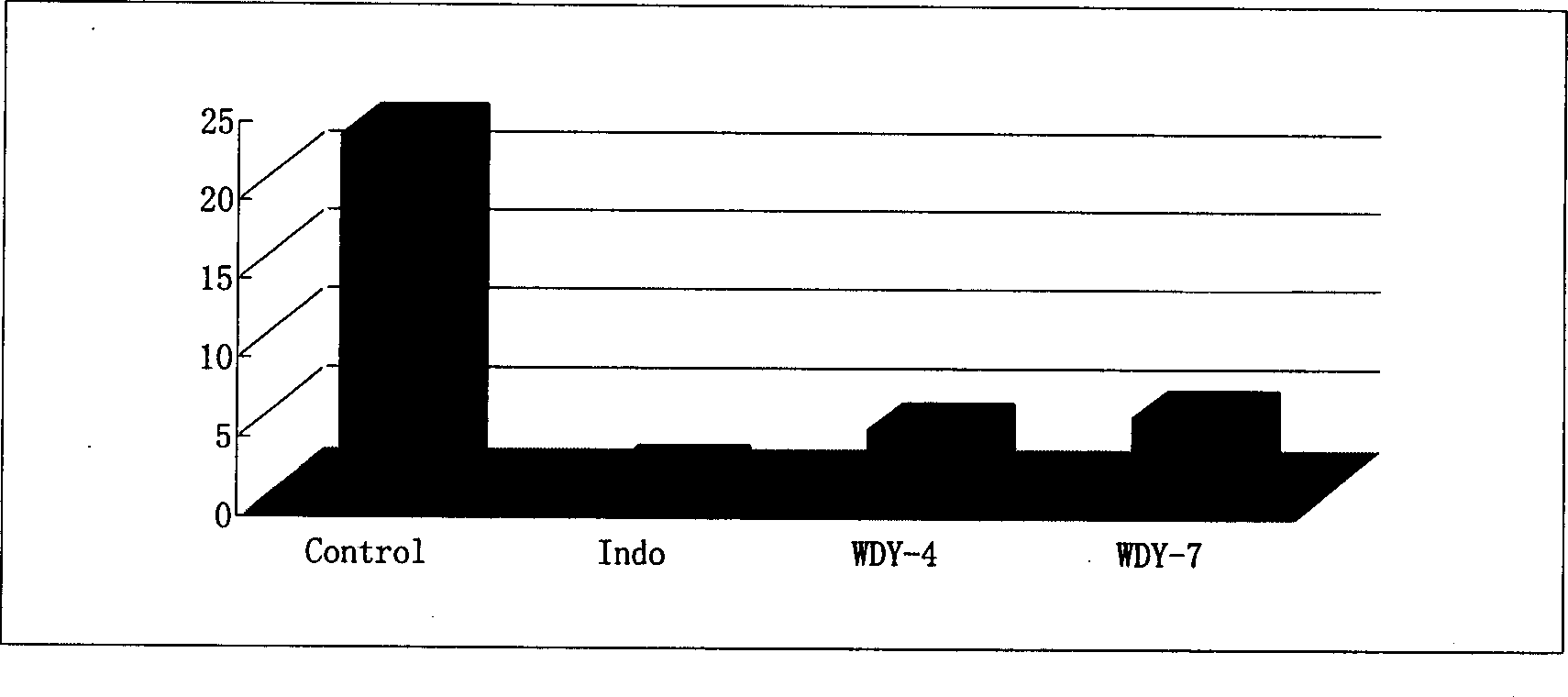
Aqueous triptolide alcohol derivative with high immunesuppressive activity and its application
A technology of triptolide and its derivatives, applied in the treatment of diseases related to immunosuppression, in the field of water-soluble triptolide derivatives, which can solve the problems of inability, low water solubility, poor oral effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The present invention will be described in detail in the following 12 examples. Example 1: Preparation of 12-β-thiocyanate triptolide-13-α-14-β-sodium phosphate (hereinafter referred to as WDY1)
[0040]
[0041] Under nitrogen protection, add 251 mg of T-SCN (0.600 mmol) to the three-necked flask, then add 20 ml of pyridine, and add 0.28 ml of POCl 3 (3.000 mmol), finally added 40 mg of DMAP, sealed, reacted at room temperature for 24 hours, cooled with an ice bath for hydrolysis, and adjusted the pH to 8. Concentrate to dryness under reduced pressure, dissolve the sample with acetonitrile, and remove inorganic salts. TLC detection: n-butanol: water: glacial acetic acid (10:1:1) developed, the main point is the product point WDY1, containing a very small amount of T-SCN and other by-products. Acetonitrile was dissolved, 2g of 75-300 mesh silica gel was added to mix the samples, the acetonitrile was removed, and 75-300 mesh silica gel column chromatography was used...
Embodiment 2
[0043] 31 PNMR, δppm: 4.57. Example 2: Preparation of 12-β-thiocyanato-13-α-hydroxytriptolide-14-β-succinic acid monoester (hereinafter referred to as WDY6)
[0044]
[0045] At room temperature, 80 mg of 12-β-thiocyanato-13-α-14-β-hydroxy triptolide (T-SCN) (0.191 mmol), 8 ml of pyridine, 4 ml of DMF and 4 ml of DMF were added to a 50 ml three-necked flask. 24 mg of DMAP, finally added 3.2 g of succinic anhydride (3.820 mmol), sealed and stirred for a week, poured the reaction solution into ice water, extracted with dichloromethane several times, combined with dichloromethane, checked by TLC: chloroform: methanol (10 : 1), the main point is WDY6 and a very small amount of unreacted 12-β-thiocyanato-13-α-14-β-hydroxy triptolide. Concentrate under reduced pressure, evaporate the solvent to dryness, and use H silica gel, chloroform:methanol (14:1) column chromatography to obtain pure product. The yield was 90%. Rf: 0.30. The melting point is 127-129°C. Water solubility i...
Embodiment 3
[0049] 13 CNMR, δppm: 14.3(18-C), 15.7(17-C), 16.3(16-C), 16.8(2-C), 22.2(6-C), 28.8~28.9(-CH2CH2-), 29.4( 15-C), 30.1(1-C), 35.2(10-C), 39.5(5-C), 50.9(12-C), 57.7(11-C), 59.0(8-C), 62.1(7 -C), 67.1(9-C), 70.6(19-C), 74.2(14-C), 75.6(13-C), 114.0(SCN), 123.5(3-C), 162.2(4-C) , 170.8(-CO-), 173.38~173.43(-COOH, 20-C). Example 3: Preparation of the preparation method of triptolide-14-β-disodium phosphate (hereinafter referred to as WDY 7)
[0050]
[0051] Under nitrogen protection, 180mgT (0.500mmol), 10ml pyridine were added to the three-necked flask, and 0.14ml POCl was slowly added dropwise. 3 (1.50 mmol), the dropwise addition was completed, the reaction was sealed for 2 to 3 hours, cooled in an ice bath for hydrolysis, adjusted to pH=9 with sodium bicarbonate, concentrated to dryness under reduced pressure, dissolved in methanol to remove inorganic salts. TLC check: n-butanol: water: glacial acetic acid (4:1:1), the main point is WDY7, containing a very small amoun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| water solubility | aaaaa | aaaaa |
| water solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com