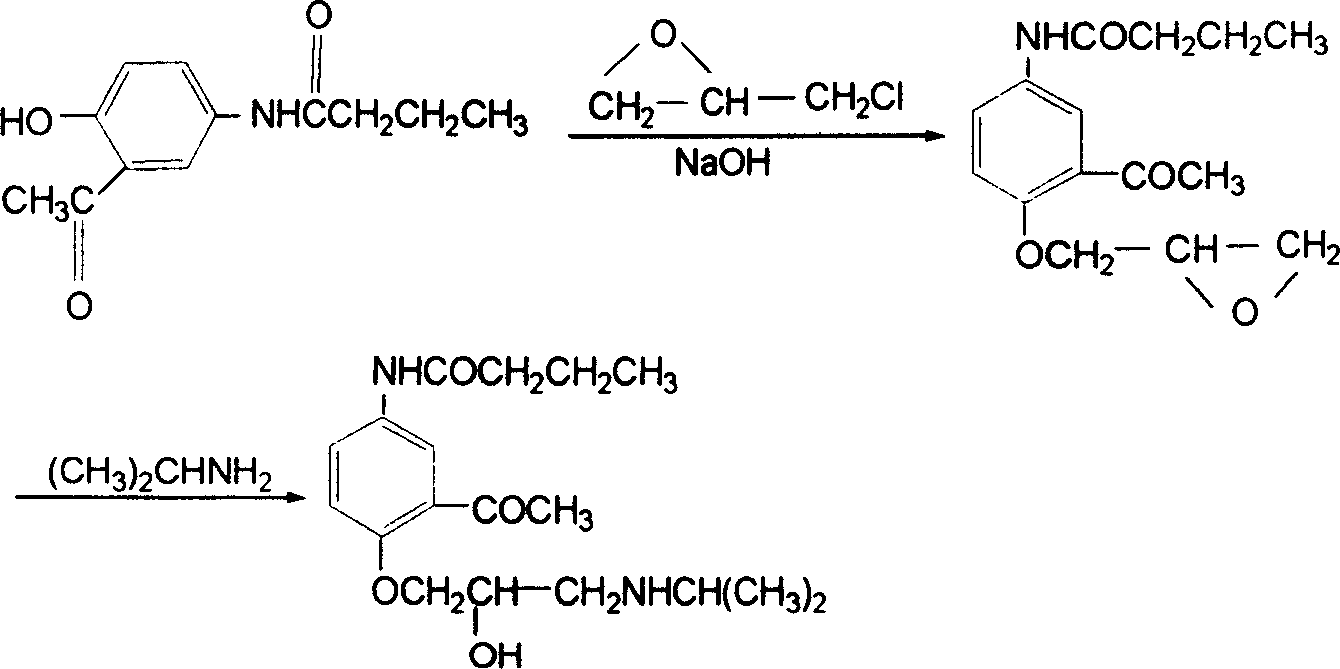
Acebutolol synthesis
A technology of butyrylolol and synthetic method, which is applied in chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve the problems of side reactions of ring-opening polymerization, difficulties in batch production, high cost, etc., and meet the reaction conditions Gentle, easy to operate, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Synthesis of 5-butanylamino-2-(2,3-epoxypropoxy)acetophenone
[0025] At room temperature 19°C, in a 500ml container, dissolve 11g of solid sodium hydroxide in 150ml of water to obtain a sodium hydroxide solution; within 2 hours, under stirring, divide 5-butyrylamide-2-hydroxy The acetophenone solid was added into the sodium hydroxide solution, and the amount of each addition was 8 g, and the next batch of 5-butyrylamino-2-hydroxyacetophenone was added after each time it was dissolved. After the 5-butyrylamino-2-hydroxyacetophenone solid was completely dissolved in the sodium hydroxide solution, 42 g of epichlorohydrin was added dropwise to the reaction system, and stirring was continued after the addition was completed; after the reaction was carried out for 10 hours, additional solids were added 6g of sodium hydroxide, 21g of epichlorohydrin was added dropwise; after stirring for 10 hours, 6g of sodium hydroxide was added again, and 21g of epichlorohydrin was adde...
Embodiment 2
[0038] (1) Synthesis of 5-butanylamino-2-(2,3-epoxypropoxy)acetophenone
[0039] At room temperature 27°C, in a 500ml reactor, dissolve 23g of solid potassium hydroxide in 300ml of water to obtain an aqueous solution of potassium hydroxide; within 1 hour, under stirring, divide 5-butyrylamide-2- The hydroxyacetophenone solid is added in the potassium hydroxide solution, and the amount of each addition is 10 g, and the next batch of 5-butyrylamide-2-hydroxyacetophenone is added after each time it is dissolved. After the 5-butyrylamino-2-hydroxyacetophenone solid was completely dissolved in the potassium hydroxide solution, 25 g of epichlorohydrin was added dropwise to the reaction system, and stirring was continued after the addition was completed; after the reaction was carried out for 8 hours, additional 12g of solid potassium hydroxide, 13g of epichlorohydrin was added dropwise; after stirring for 8 hours, 12g of solid potassium hydroxide was added again, and 13g of epichlor...
Embodiment 3
[0045] (1) Synthesis of 5-butanylamino-2-(2,3-epoxypropoxy)acetophenone
[0046] At room temperature 14°C, in a 500ml container, dissolve 14g of solid sodium hydroxide in 250ml of water to obtain a sodium hydroxide solution; within 1 hour, under stirring, divide 5-butyrylamide-2-hydroxy The acetophenone solid is added in the sodium hydroxide solution, and the amount of each addition is 5 g, and the next batch of 5-butyrylamino-2-hydroxyacetophenone is added after each time it is dissolved. After the 5-butyrylamino-2-hydroxyacetophenone solid was completely dissolved in the sodium hydroxide solution, 44 g of epichlorohydrin was added dropwise to the reaction system, and stirring was continued after the addition was completed; after the reaction was carried out for 12 hours, additional solids were added 7g of sodium hydroxide, 22g of epichlorohydrin was added dropwise; after stirring for 12 hours, 7g of solid sodium hydroxide was added again, and 22g of epichlorohydrin was added...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com