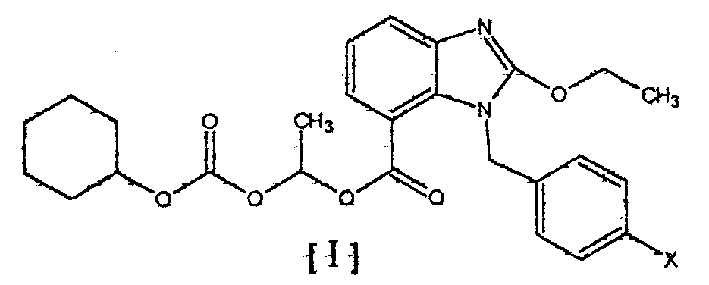
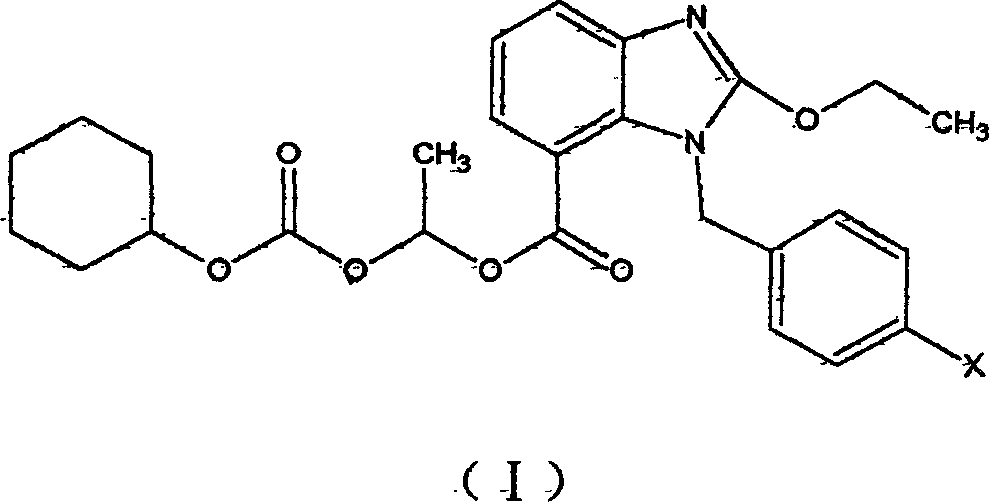
Ester compounds of bezimidazole and their preparations and uses in preparation of medicinal compound
A technology for ester compounds and benzimidazoles, applied in the field of preparing medicinal compounds candesartan medoxomil, can solve the problems of long synthetic route, complicated operation, adding auxiliary steps of adding protective group and deprotecting group, etc. Simple operation, great flexibility and applicability, cost-reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
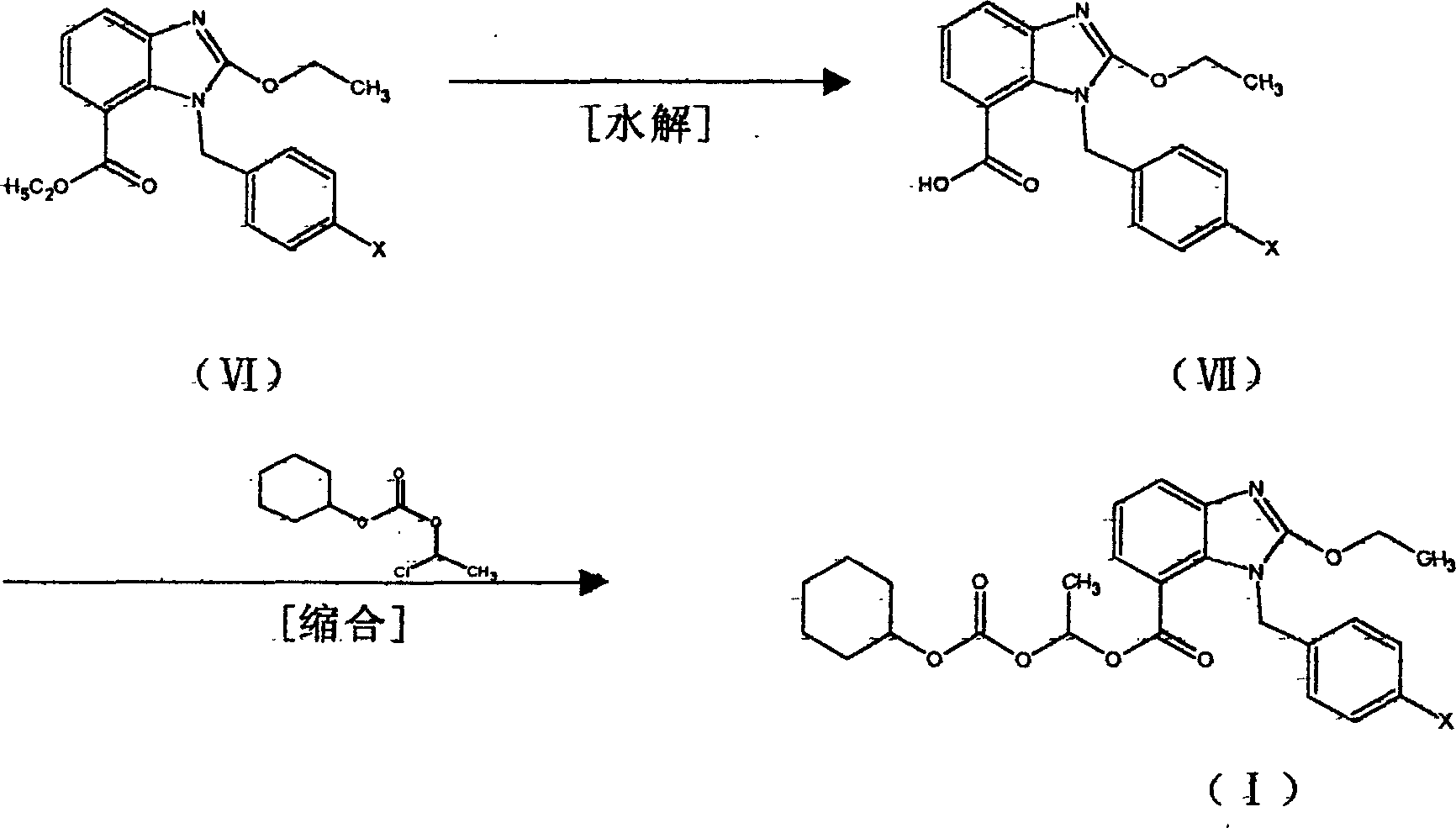
[0031] One of the preparation methods of ethyl 1-(p-bromophenyl)methyl-2-ethoxybenzimidazole-7-carboxylate (VI)
[0032] a) Preparation of 2-tert-butoxycarbonylamino-3-nitrobenzoic acid ethyl ester (II)
[0033] 24 g (0.1 mol) of ethyl 2-carboxy-3-nitrobenzoate, 20 ml of thionyl chloride and 100 ml of toluene were sequentially added into the reaction flask, and heated under reflux for 3 hours. The reaction mixture was concentrated to dryness. The obtained acid chloride was dissolved in 100 ml of chloroform, and a solution of 10 g (0.15 mol) of sodium azide (0.15 mol) and 50 ml of DMF was added dropwise with stirring. After the addition was complete, the reaction was continued for 3 hours. Add 500ml of water to the reaction mixture, place in a separatory funnel, separate the organic layer, extract the water layer with chloroform, wash the organic layer with water, dry, and recover the solvent by distillation. The residue was dissolved in tert-butanol, the solution was gradual...
example 2
[0041] The second preparation method of 1-(p-bromophenyl)methyl-2-ethoxybenzimidazole-7-carboxylic acid ethyl ester (VI)
[0042] a) Preparation of ethyl 2-ethoxybenzene-1H imidazole-7-carboxylate (VIII) (ie [1-1])
[0043] 36 g (0.1 mol) of ethyl 2,3-diaminobenzoate, 60 g of ethyl orthocarbonate, and 5 g of acetic acid were sequentially added into the reaction flask, and stirred and reacted at 80° C. for 5 hours. The reaction mixture was concentrated, and the concentrate was dissolved in ethyl acetate, washed successively with aqueous sodium bicarbonate and water. The solvent was distilled off, and the residue was recrystallized from ethyl acetate-benzene to obtain 16 g of a yellow solid, yield 70%, mp.125-130°C
[0044] b) Preparation of 1-(p-bromophenyl)methyl-2-ethoxybenzimidazole-7-carboxylic acid ethyl ester (VI)
[0045] Add 200 ml of acetonitrile to 23.4 g (0.1 mol) of the intermediate 2-ethoxybenzene-1H-imidazole-7-carboxylic acid ethyl ester (VIII) prepared above, ...
example 3
[0047] Preparation of 1-(p-bromophenyl)methyl-2-ethoxybenzimidazole-7-carboxylic acid (VII)
[0048] Add 40 g of ethyl 1-(p-bromophenyl)methyl-2-ethoxybenzimidazole-7-carboxylate (VI) obtained in the above-mentioned manner to 150 ml of 1N NaOH and 100 ml of ethanol, and stir at reflux for reaction 6 Hours, concentrated, 200ml of water was added to the concentrate, extracted with ethyl acetate, the aqueous layer was adjusted to pH 3-4 with 1N HCl to precipitate crystals, which were recrystallized in ethyl acetate-ethanol to obtain 16g of colorless crystal product (VII), The calculated yield was 42%, mp. (melting point) 175-180°C.
[0049] Element composition Theoretical value % Measured value (%)
[0050] C 17 h 15 BrN 2 o 3 C54.41 54.28
[0051] 375.217 H4.03 4.15
[0052] N7.46 7.47
[0053] Br21.32 21.06
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com