Artificial combined antistaphylococcus engineering multipeptide and its preparation method
A Staphylococcus, engineering technology, applied in the field of artificially combined antibacterial engineering polypeptide and its preparation, can solve the problem of only function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
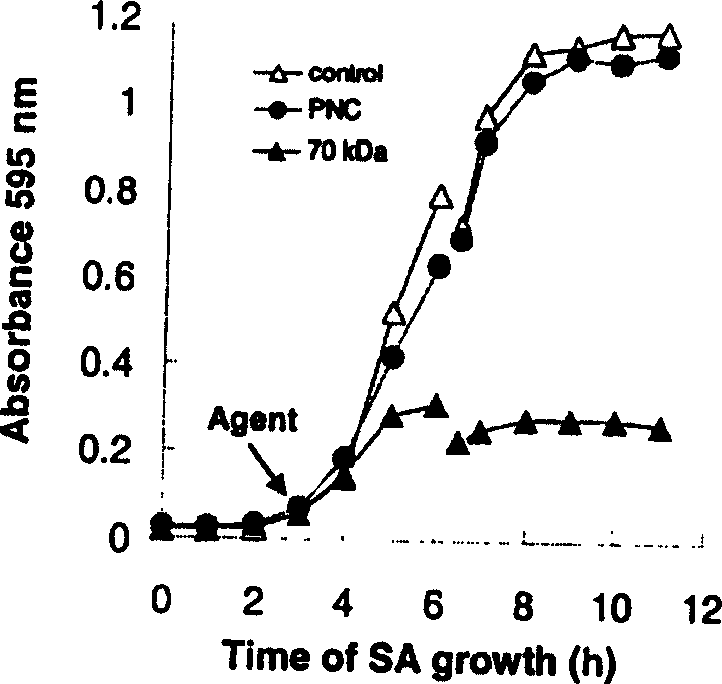
[0015]In this example, the Agr D signaling polypeptide of Staphylococcus aureus is linked to the carboxyl terminus of colistin Ia to prepare a plasmid for artificially combining anti-Staphylococcus aureus engineering polypeptide, and the mutated plasmid is transfected into In the engineered bacteria with plasmids, the bacteria were mass-proliferated (4L FB medium, 225rpm, 37°C; 6h), and the bacterial cells were centrifuged (4°C, 6000g, 20min). Take 4°C, 50mM boric acid buffer + 2mM EDTA + 2mM DTT 50-80ml suspension cells, ultrasonic disruption (4°C, 40W, 1-2min), high-speed centrifugation to break up the cells (4°C, 50000-70000g, 1.5h), take the supernatant and add streptomycin sulfate to precipitate DNA, 4 ℃, 50mM boric acid buffer + 2mM EDTA + 2mM DTT2L dialyzed overnight, the supernatant was loaded on a CM ion exchange column, and after elution at 4 ℃, 0.3M NaCl + 50mM boric acid buffer, the anti-Staphylococcus aureus engineering polypeptide was obtained. Molecular weight 6...
Embodiment 2
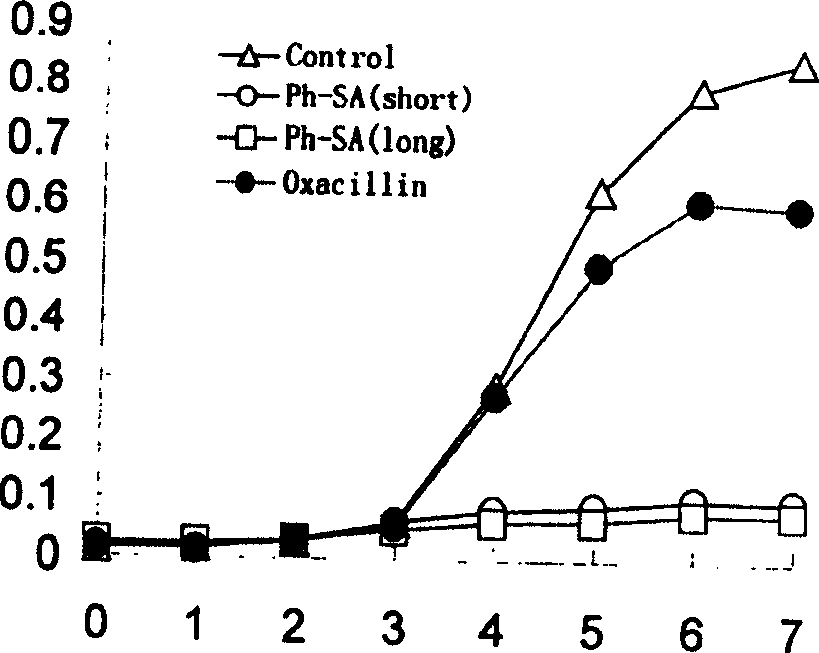
[0017] The wooden embodiment is that the Agr D signaling polypeptide of Staphylococcus aureus is connected to the carboxyl end of the colicin Ia water-based pore structure domain, and the plasmid is prepared, and then through the technical steps used in the example one, a smaller than the example one is obtained. Anti-Staphylococcus aureus engineered polypeptide with a molecular weight of 20,000. In order to confirm the antibacterial ability of the engineering polypeptide, 5 μl (10 8 CFUS / ml) into 10ml of LB medium with glucose, incubated at 225rpm, 37°C for three hours, then added the antibacterial engineering polypeptide (final concentration 0.5μg / ml), continued to incubate at 225rpm, 37°C, and sampled every hour Spectrophotometer ((A595nm) colorimetric test, with oxacillin (Oxacillin, final concentration 5 μ g / ml) and Staphylococcus aureus without adding any drug as contrast (see attached figure 2 ), the results showed that: after the staphylococcus aureus in the control ...
Embodiment 3
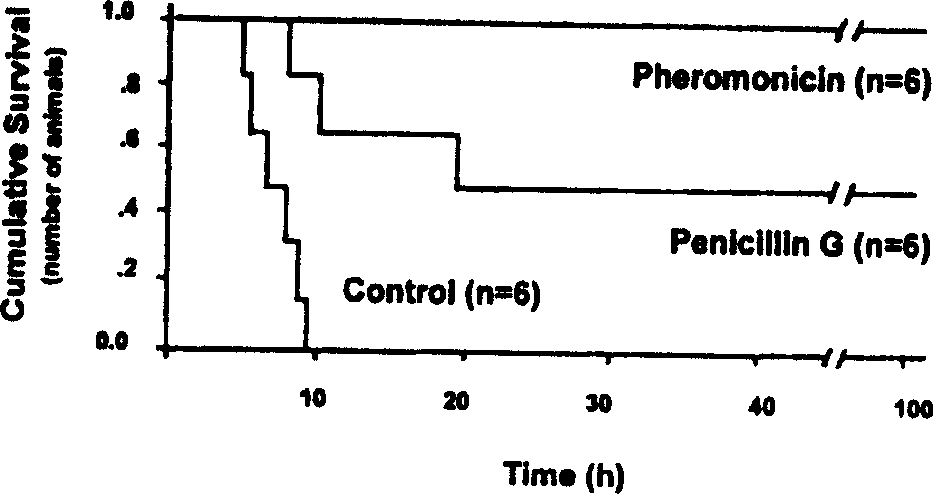
[0019] In this example, according to the above technical route, we conducted an in vivo bactericidal experiment on the prepared 20,000 molecular weight anti-S. aureus engineering polypeptide. Eighteen Kunming mice, half male and half female, weighing 25-30 g, were intraperitoneally injected with penicillin-resistant Staphylococcus aureus (ATCC 29213, American standard strain, the lethal dose was calibrated and injected by the Pharmacology Office of Sichuan Antibiotic Research Institute, National Drug Inspection Bureau) 1 One hour later, they were randomly divided into 3 groups, (1) intraperitoneal injection of 0.5ml 0.9% normal saline, once every 6 hours, a total of 4 times as the control group (n=6); (2) intraperitoneal injection of 2.5mg penicillin G sodium, every 6 hours Once an hour for a total of 4 times (n=6); (3) Inject 2 mg of 20,000 molecular weight antibacterial engineering polypeptide into the intraperitoneal cavity, once every 6 hours for a total of 4 times (n=6). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com