Gene of streptokinase, recombination protein and preparation method
A thrombolytic enzyme and gene technology, applied in the field of preparation of the source strain of the thrombolytic enzyme gene, the thrombolytic enzyme gene and the protein produced by its expression, can solve the problem of high cost, high treatment cost, and increased burden on patients and society and other issues to achieve the effect of low cost, cost reduction and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Example 1 Preparation process of Bacillus subtilis QK02
[0123] (1) Take rice leaves and heat-treat them at 85°C for 10 minutes, wrap the sterilized soybeans with them, cultivate them at 42°C for 24 hours, and then use the culture medium (soybean extract contains 0.5% salt, 0.5% Na 2 HPO 4 , 2% liquefied starch) soak the soybeans that have been cultivated, and make the nutrient solution of soaked soybeans for 10 -2 -10 -6 Dilute, spread on a nutrient agar plate, grow colonies on the plate after inverting at 37°C.
[0124] (2) The obtained colonies were respectively inoculated in 5 ml of culture solution and cultured at 37° C. and 250 rpm for 24 hours, and then centrifuged at 6000 rpm for 5 minutes. Take 20 μl of supernatant to measure thrombolytic enzyme activity on a fibrin plate, and then select the strain with the highest enzyme activity.
Embodiment 2
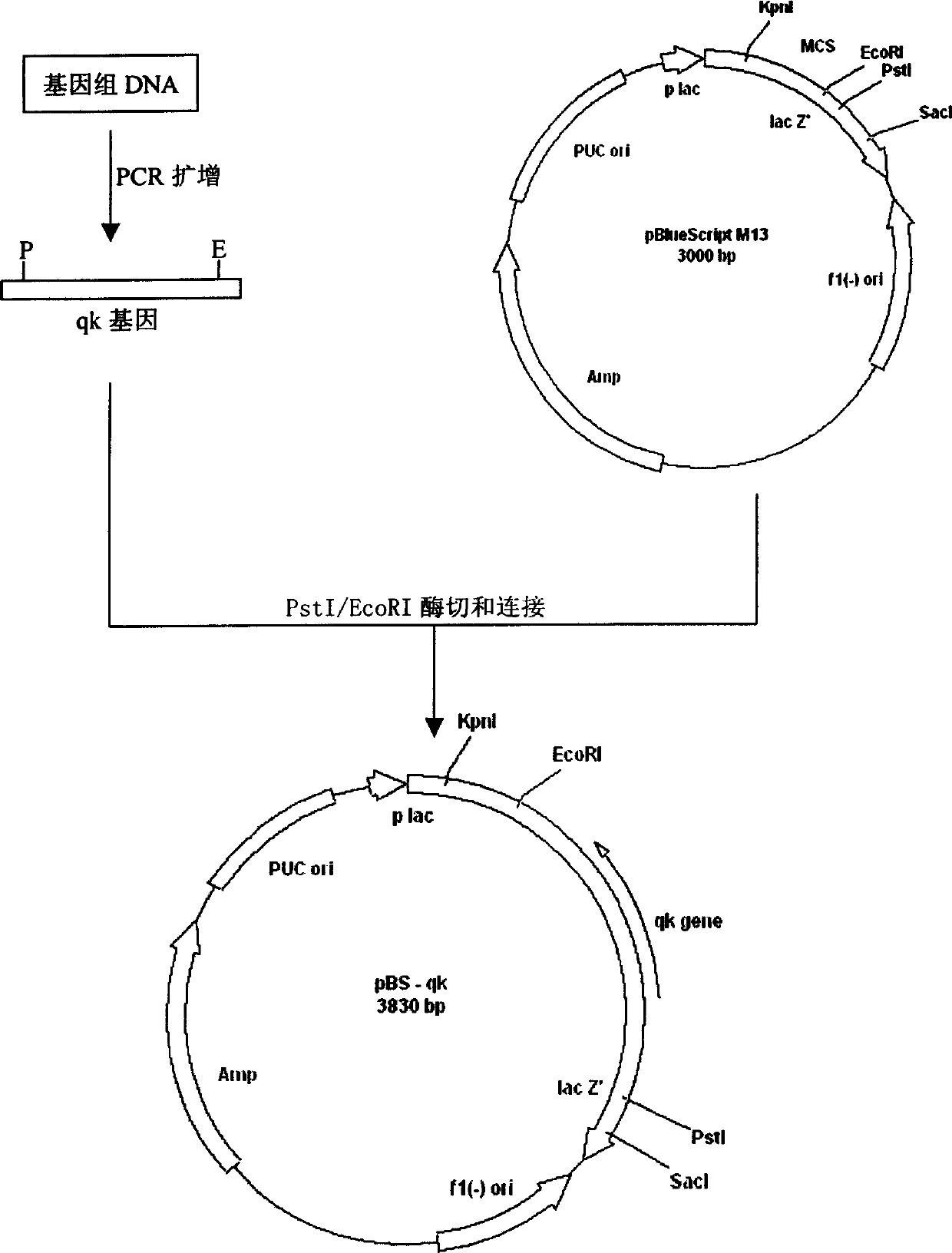
[0125] Example 2 PCR amplification and cloning and sequence determination of gene qk encoding thrombolytic enzyme QK
[0126] (1) Genomic DNA extraction of Bacillus subtilis QK02 was performed according to a conventional method (Saito, H. et al, 1963, Biochim. Biophys. Acta 72: 619-629).
[0127] (2) The primers for PCR amplification were designed according to the N-terminal 20 amino acids of thrombolyticase QK (AQSVPYGISQIKAPALHSQG, sequenced at Hunan Normal University) and the C-terminal sequence of the Bacillus subtilis aprN gene. The size of the amplified fragment was 828bp.
[0128] Primer P8: 5’CGC CTG CAG ATG GCG CAA TCT GTT CCT TAT GGC ATT
[0129] Primer P9: 5’GCG GAA TTC TTA CTA TTA TTG TGC AGC TGC TTG
[0130] The above primers were synthesized by Shanghai Jikang Bioengineering Company. In the 50 μl PCR amplification reaction system: 9 μl Bacillus subtilis genomic DNA as a template; the total concentration of each primer is 0.8 pmol / μl; dNTP 1 mM; 10×PCR buffer 5...
Embodiment 3
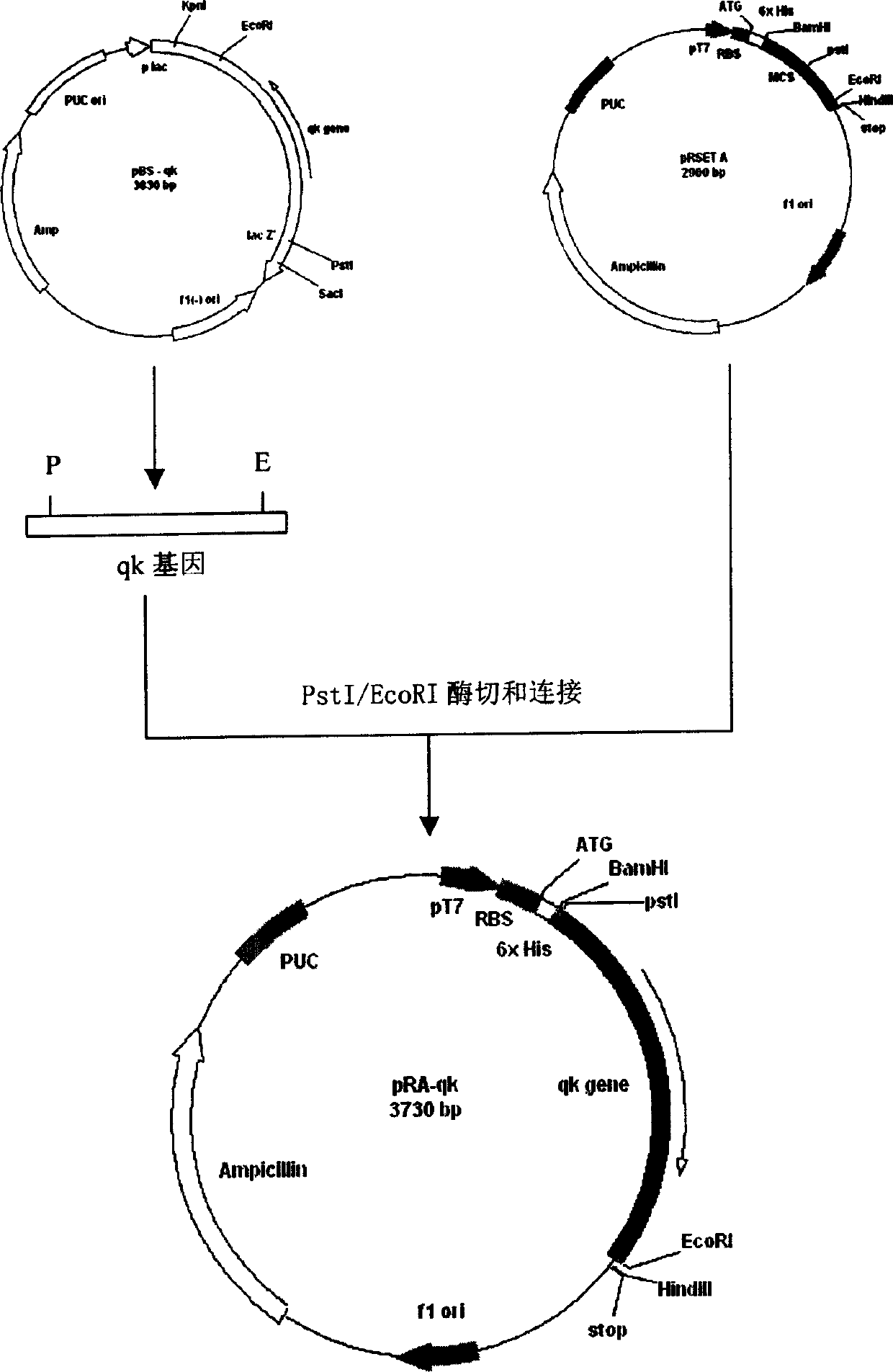
[0133] Construction and expression of the recombinant expression plasmid of the gene qk of embodiment 3 encoding thrombolytic enzyme QK
[0134] (1) Use PstI and EcoRI to excise and recover the qk gene from the pBS-qk plasmid, and add T to the pRSET A plasmid DNA that has been digested and recovered by PstI / EcoRI 4 DNA ligase was ligated at 18°C for 10 hours, and the ligation product was transformed into CaCl 2 E.coli BL21 (DE3) competent cells prepared by the method were spread on plates to screen recombinants, plasmids were extracted by rapid method, and positive recombinants were further identified by PstI / EcoRI double enzyme digestion. See Figure 2 for the specific process.
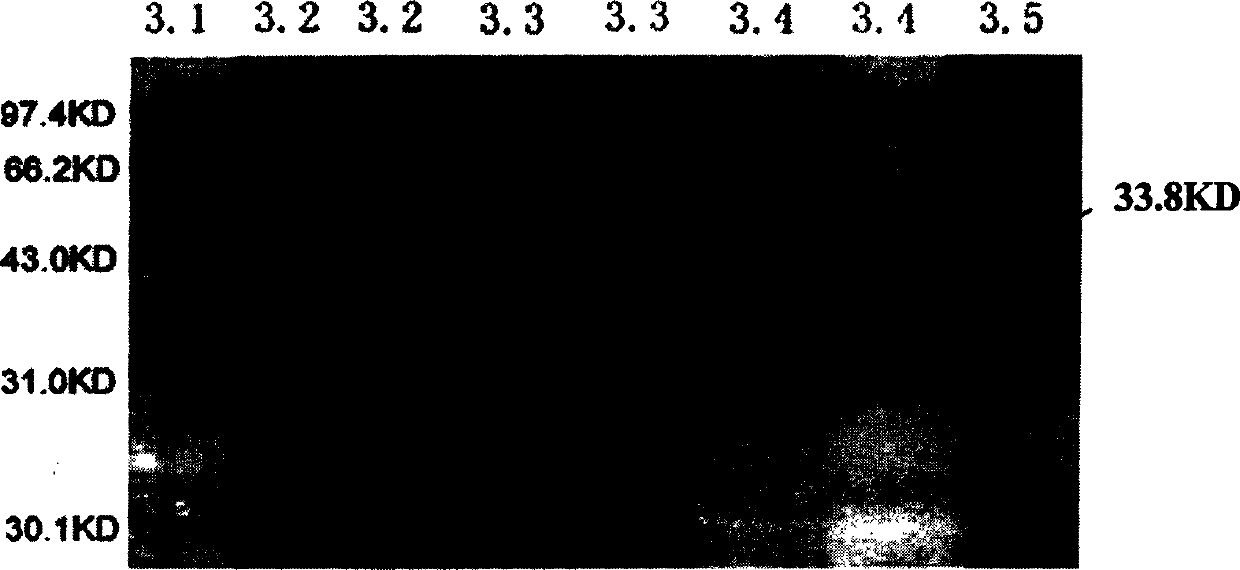
[0135] (2) The expression vector pRSET is controlled by the T7 promoter, and the expression plasmid constructed with this vector can be induced and expressed by adding IPTG at 37°C or at room temperature. Pick a single positive colony and inoculate it in 2ml of LB medium, place it on a shaker at 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com