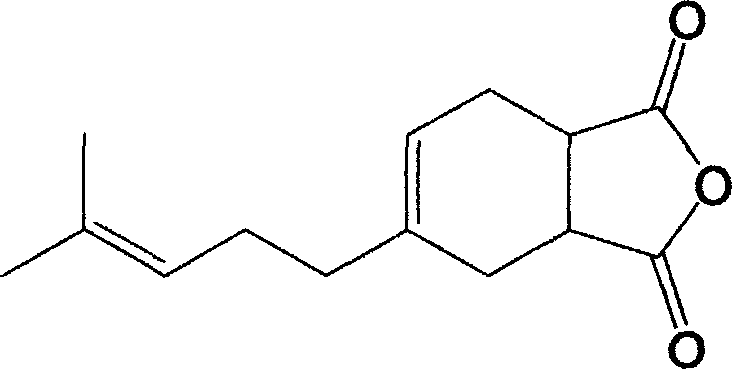
Novel purpose of 4-(4-methyl-3-pentenyl)-4-cyclohexene-1, 2-diacid anhydride
A technology based on pentenyl and cyclohexene, which is applied in the field of 4--4-cyclohexene-1, can solve the problems of complex additive structure, increased solder composition, complex composition, etc., and achieves easy cleaning, less residue, Synthetic easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
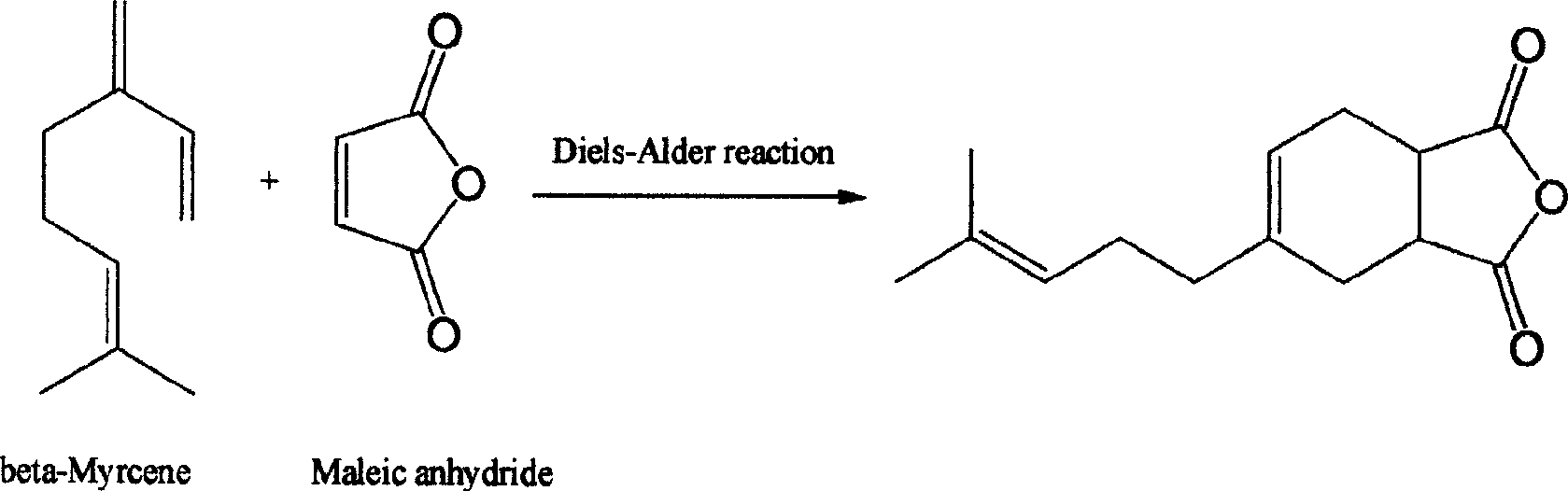
[0012] The specific preparation method is: taking the monoterpene compound β-myrcene and maleic anhydride as raw materials, under heating, after dripping β-myrcene to maleic anhydride, intermolecular Diels-Alpha Diels-Alder cycloaddition reaction. The product is obtained by collecting fractions at 155-160°C / 3mmHg by vacuum distillation. The crude reaction product and the vacuum distillation product after the reaction were respectively used as welding fluxes for welding activity test.
[0013] The raw material β-myrcene is an important fragrance and the basic raw material of synthetic fragrance. It can be easily prepared in large quantities from β-pinene, one of the main components of turpentine oil.
[0014] Welding activity test
[0015] The welding activity test follows the method stipulated by JIS-Z-3197 in Japan. The spread rate of solder when the new soldering flux is used on the copper plate is measured. Compared with the reference material rosin, the larger the spread rate,...
Embodiment 1
[0016] Example 1 (Preparation of 4-(4-methyl-3-pentenyl)-4-cyclohexene-1,2-dianhydride)
[0017] In a four-necked flask equipped with a thermometer, a dropping funnel, a mechanical stirrer, and a condenser, add maleic anhydride (1.0 times mole calculated as β-myrcene) and heat to dissolve the maleic anhydride, and control it to 65- At 70°C, β-myrcene (β-myrcene content 75%) was added dropwise from the dropping funnel. After dripping, react at 65-70°C for 4 hours. The crude product obtained by distilling off low-boiling components is used as solder A for soldering test. The product 4-(4-methyl-3-pentenyl)-4-cyclohexene-1,2-dianhydride was collected by vacuum distillation to collect the fraction at 155-160℃ / 3mmHg, and the structure was determined by nuclear magnetic resonance as Flux B is used for welding test.
Embodiment 2
[0018] Example 2 (Measurement of Welding Activity)
[0019] The soldering activity test follows the method specified by JIS-Z-3197 in Japan, using H60A type solder with a diameter of 1.6mm, and rosin as a comparative substance.
[0020] The 20mm*20mm*0.3mm copper plate was washed with isopropanol and heated and oxidized at 150°C for 1 hour. Weigh 0.250mg±0.01 solder and roll it into a circle shape and place it on the copper plate. Place the solder sample to be measured in the center of the circular solder. The sample weight is 25mg±5. Put the above copper plate on the heating plate and control the heating for 4 minutes. When the temperature reaches 200°C, cut off the power, and after cooling, use monoterpenes such as limonene and pinene as a solvent to wash the copper plate to remove. Measure the thickness and diameter of the expanded solder. Use the following formula to calculate the expansion rate of the solder. The larger the expansion rate value is Means the higher the activit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com