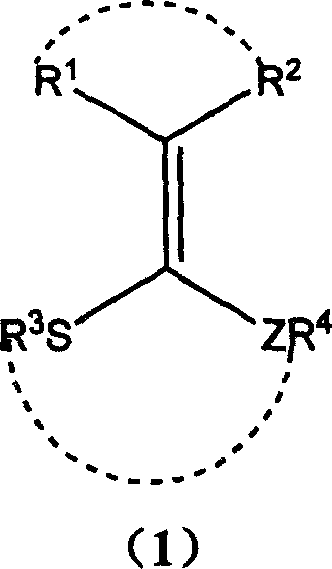
Application of ketene thioacetal derivatives as thioalcohol substituted reagent
A technology of thioketal and derivatives, applied in the application field of environment-friendly organic reagents in organic synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
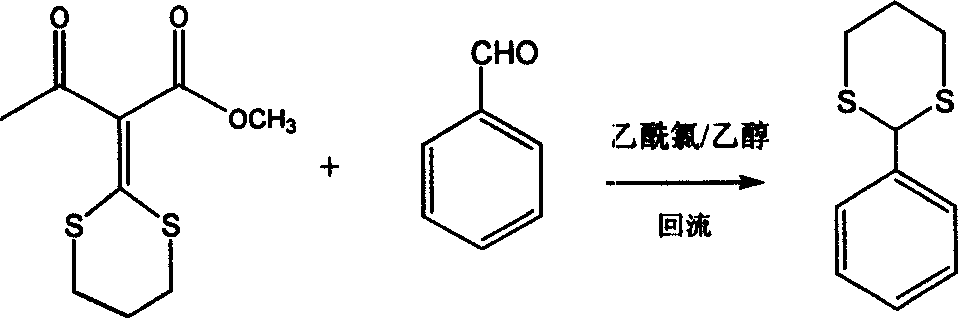
[0040] In a 50 ml round bottom flask, add 0.648 g (2 mmol) of 2-(1,3 propylenedithio) methylene-butyric acid methyl ester-3-ketone and 2 mmol of benzaldehyde, then, add 10 milliliters of ethanol was stirred, and then 0.712 milliliters of acetyl chloride was added, heated to reflux, monitored by TLC until the benzaldehyde disappeared, the ethanol was distilled off under reduced pressure, and column chromatography gave 2-phenyl-[1,3]dithiane as a white solid. Yield: 84.1%. The reaction is shown in the following formula:
[0041]
Embodiment 2
[0043] In a 50 ml round bottom flask, add 0.404 g (2 mmol) of 3-(1,2-ethylenedithio)methylene-2,4-pentanedione and 0.302 g (2 mmol) of 4- Nitrobenzaldehyde, then, add 10 milliliters of methanol, stir, then add 0.712 milliliters of acetyl chloride, heat to reflux, TLC monitors until 4-nitrobenzaldehyde disappears, evaporate methanol under reduced pressure, and column chromatography gives a white solid to obtain 2 -(4-nitrophenyl)-[1,3]dithiapentane 0.380 g, yield 83.5%. The reaction is shown in the following formula:
[0044]
Embodiment 3
[0046] In a 50 ml round bottom flask, add 0.432 g (2 mmol) of 3-(1,3-propylenedithio)methylene-2,4-pentanedione, 0.212 g (2 mmol) of benzaldehyde and 0.240 grams (2 mmol) of acetophenone, then add 10 milliliters of methanol, stir, then add 0.5 milliliters of acetyl chloride, heat to reflux, TLC monitors the reaction for 3 hours, evaporates methanol under reduced pressure, and column chromatography gives white solid 2 -0.383 g of phenyl-[1,3]dithiane, the yield was 91.3%, and 0.218 g (95%) of acetophenone was recovered at the same time. The reaction is shown in the following formula:
[0047]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com