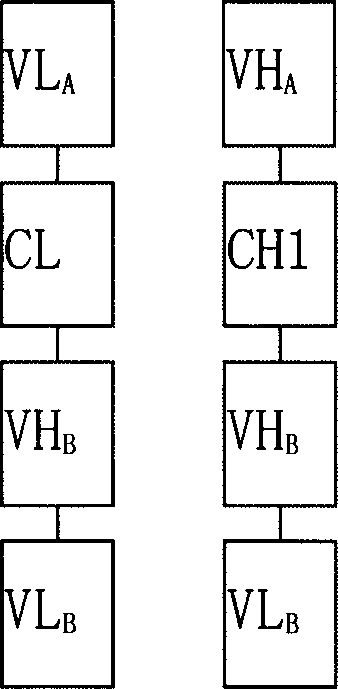
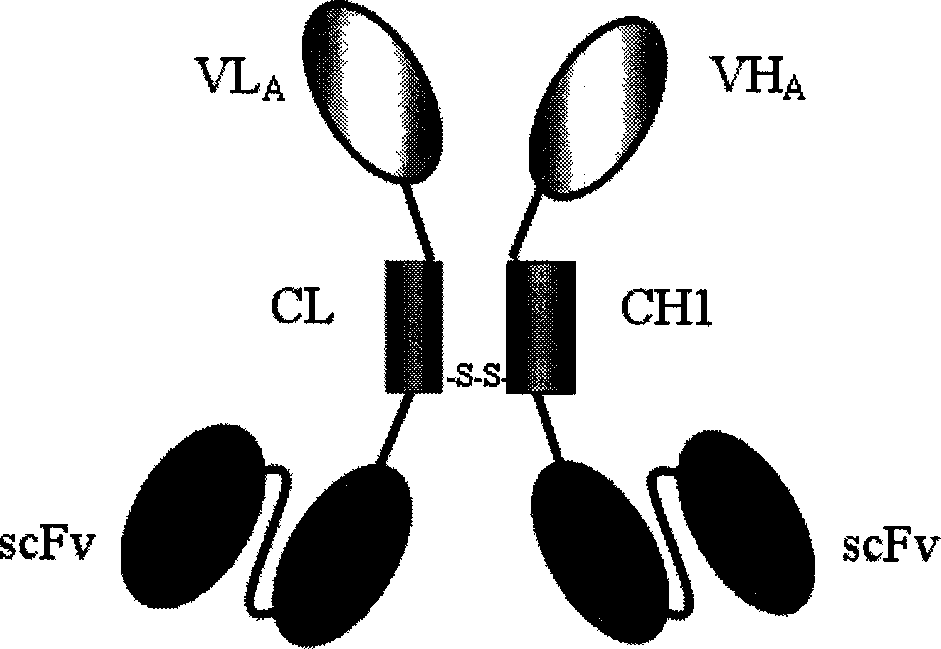
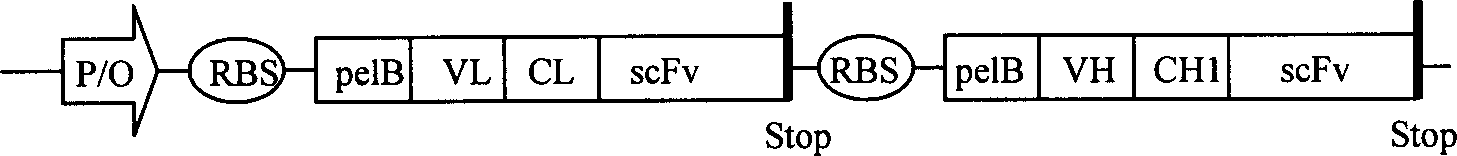
Trivalent bispecific antibody and its preparation process and use
A bispecific antibody, antibody technology, applied in the direction of antibodies, chemical instruments and methods, specific peptides, etc., can solve the problems of low target molecule affinity and poor guiding effect, and achieve enhanced effector function, reduced concentration, and alleviated side effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of anti-Her2 / neu×anti-CD16 trivalent BsAb
[0050] 1. Materials
[0051] Mouse anti-human CD16 monoclonal antibody light chain, heavy chain variable region genes (VL, VH) were cloned from hybridoma cell B88-9, commercially available (refer to "Molecular Cloning" (Science Press, second edition) for experimental methods); mouse anti-P185 erbB2 The VL and VH genes of the monoclonal antibody were cloned from hybridoma cells Her2 (commercially purchased), and the scFv was synthesized according to conventional methods. editor-in-chief); human IgG1 heavy chain first constant region CH1 and κ chain constant region CL genes were provided by Dr. Tong Yigang, Academy of Military Medical Sciences (GenBank AF027159, X95747); Escherichia coli strains JM109, BL21(DE3), prokaryotic expression vector pET22b(+) It is a standard strain, commercially available; highly expressed P185 erbB2 The human breast cancer cell line SK-BR-3 is the standard strain (ATCC Number...
Embodiment 2
[0067] Example 2 FACS analysis and detection of anti-Her2 / neu×anti-CD16
[0068] Activity detection of trivalent BsAb
[0069] 1. Materials
[0070] Same as embodiment one.
[0071] 2. Methods and results
[0072] 1. FACS analysis of the binding activity of BsAb to SK-BR-3 cells
[0073] SK-BR-3 cells were digested with 0.02% EDTA and washed with cold PBA (PBS, 2% BSA, 0.1% NaN 3) and washed twice by centrifugation; incubated with BsAb (0.1mg / L) at 0°C for 30min; washed twice by cold PBA, incubated with FITC-GAH by shaken at 0°C for 30min; washed by centrifuge twice with cold PBA, and resuspended cells in PBS , analyzed with a Becton-Dickenson FACsort instrument. The results show that BsAb can specifically combine with intact SK-BR-3 cells, and the relative average fluorescence intensity is 92.08, which is 25.4 times that of the negative control, see Figure 12 .
[0074] 2. FACS analysis of the binding activity of BsAb to CD16 surface ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com