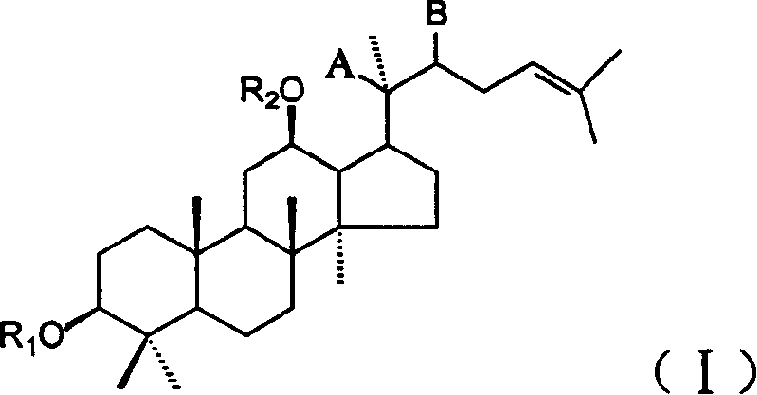
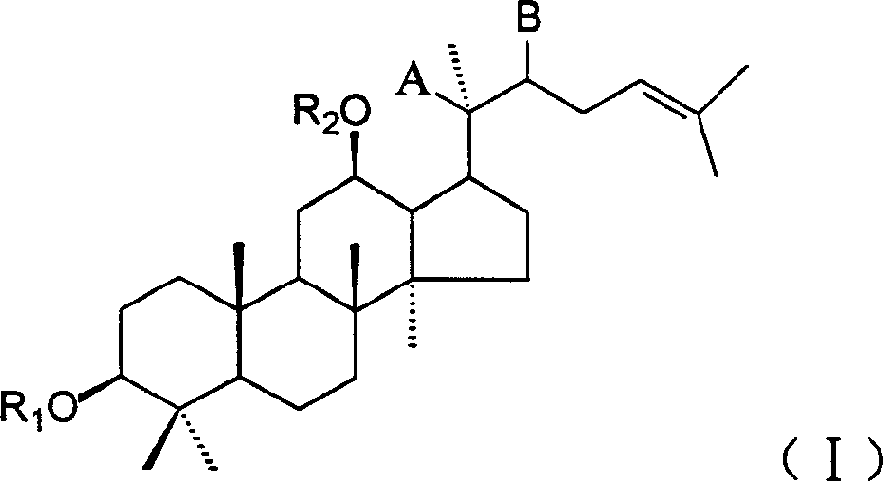
20(SO)ortho ginseng diol derivative, medicinal composition containing them and its application
A technology of protopanaxadiol and its derivatives, which is applied in the direction of drug combination, medical preparations containing active ingredients, drug delivery, etc., to achieve the effect of reducing the activity of strong antiviral and anticancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0055] Preparation of 20(S)-protopanaxadiol-12β-monomethyl ether and 20(S)-protopanaxadiol-3β, 12β-dimethyl ether
[0056] 0.38g 20(S)-protopanaxadiol was dissolved in 10mL DMF, and 0.10g sodium hydride (60%) was added. After the sodium hydride is completely dissolved, add 0.20 mL of methyl iodide, heat to 70°C, and react for 5 hours. An appropriate amount of water was added to the reaction solution, and a white solid was precipitated. The precipitated solid was filtered and dried to obtain 0.70 g. Silica gel column chromatography, eluted with chloroform, to obtain two components: component 1 is 0.27g oily substance, namely 20(S)-protopanaxadiol-3β, 12β-dimethyl ether; component 2 is 0.19g white The solid is 20(S)-protopanaxadiol-12β-monomethyl ether.
[0057] R-OCH 3
[0058] 1 H-NMR (CDCl 3 )δ: 0.79, 0.88, 0.98, 1.11 (18Hs×4+CH 3 ),, 1.69, 1.82 (6Hs, s, ), 3.35 (3H, s, -O-CH 3 ),
[0059] 5.14 (1H,t,=C-H)
[0060] 13 C-NMR (CDCl 3 )δ: 130.77, 125.50 (-C=C-), 8...
preparation Embodiment 2
[0065] Preparation of 20(S)-protopanaxadiol-12β-monoethyl ether and 20(S)-protopanaxadiol-3β, 12β-diethyl ether
[0066] 0.38g 20(S)-protopanaxadiol was dissolved in 10mL DMF, and 0.10g sodium hydride (60%) was added. After the sodium hydride is completely dissolved, add 0.20 mL of bromoethane, heat to 70°C, and react for 5 hours. An appropriate amount of water was added to the reaction solution, and a white solid was precipitated. The precipitated solid was filtered and dried to obtain 0.70 g. The crude product was purified by column chromatography and eluted with chloroform to obtain two components: component 1 was 0.13g oily substance, that is, 20(S)-protopanaxadiol-3β, 12β-diethyl ether; component 2 was 0.20g white The solid is 20(S)-protopanaxadiol-12β-monoethyl ether.
[0067] R-OCH 2 CH 3
[0068] 1 H-NMR (CDCl 3 )δ: 0.67, 0.81, 0.84, 0.88, 0.90, 0.98 (18H, s×6, +CH 3 ), 1.56, 1.64 (6H, s, s, ), 1.07 (3H, t, -CH 3 ), 3.01, 3.71 (2H, q, q, -OCH 2 -), 5.12 (=...
preparation Embodiment 3
[0074] Preparation of 20(S)-protopanaxadiol-12β-n-pentyl ether
[0075] 0.38g 20(S)-protopanaxadiol was dissolved in 10mL DMF, and 0.10g sodium hydride (60%) was added. After the sodium hydride is completely dissolved, add 0.38g (0.31mL) n-pentane bromide, heat to 70°C, and react for 5 hours. Cool to room temperature, add 50mL of water, an oily substance precipitates, and stand overnight. The supernatant was decanted, washed once with water, the oil was extracted with chloroform, and the extract was dried with anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure to obtain 1.05 g of a light yellow oil. The crude product was purified by silica gel column chromatography and eluted with chloroform to obtain 0.38 g of the title product as a pale yellow liquid.
[0076] 1 H-NMR (CDCl 3 )δ: 0.78, 0.88, 0.95, 0.98, 1.10 (18H, s×4, +CH 3 ), 0.88 (3H, t, -CH 3 ), 1.57, 1.62 (6H, s, s, ), 5.15 (1H, t, =C-H), 8.01 (1H, s, -OH), 5.60 (1H, broad, -OH) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com