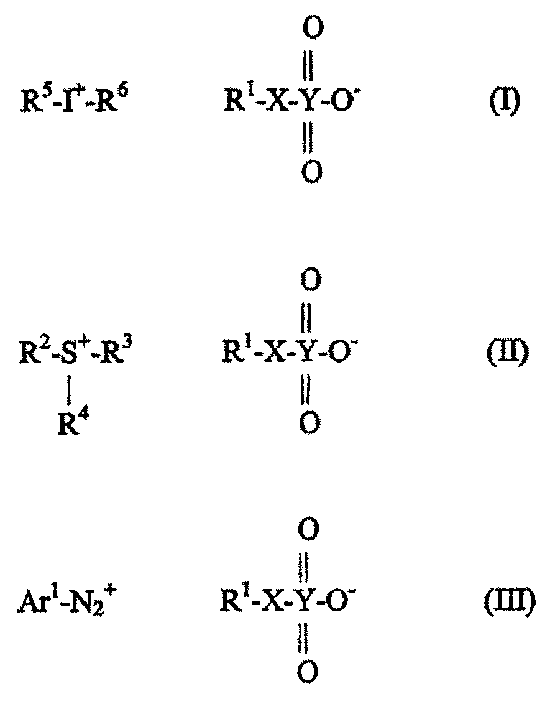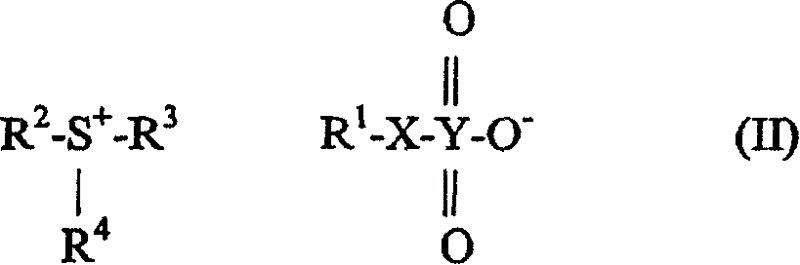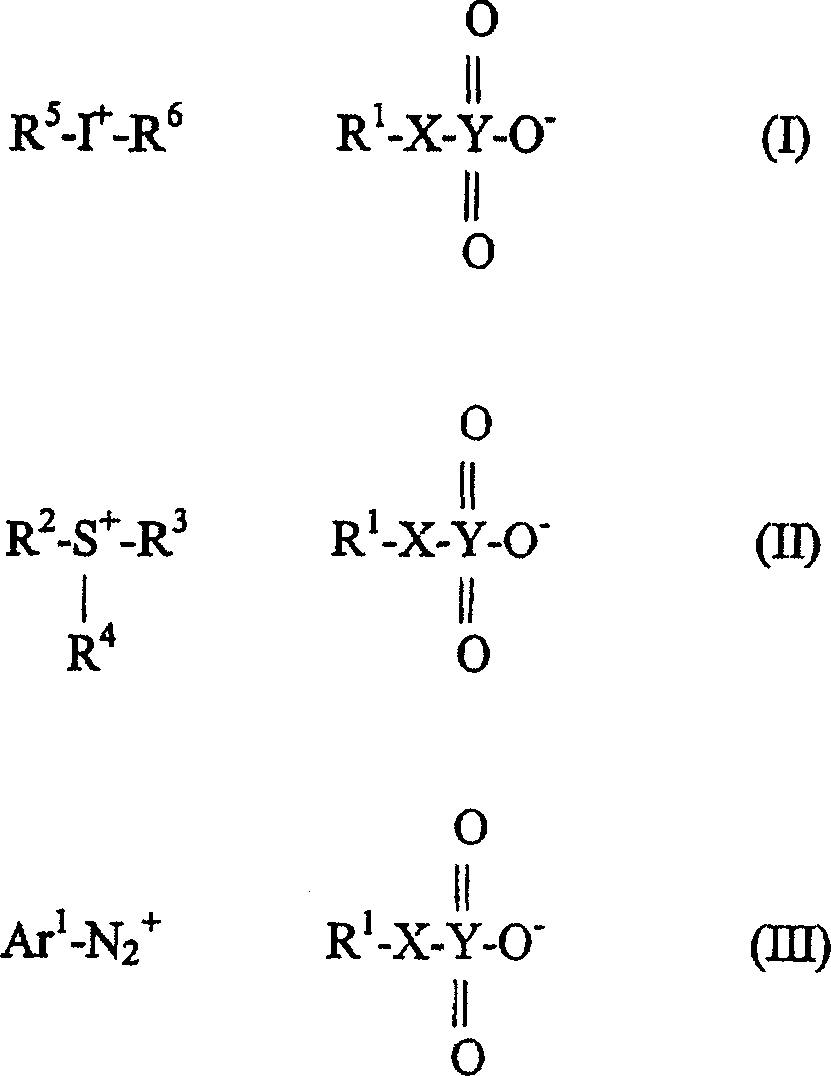Selected acid generating agents and their use in processes for imaging radiation-sensitive elements
An acid generator, photosensitive element technology, applied in photosensitive materials for optomechanical equipment, other chemical processes, photoengraving process of patterned surface, etc. Photographic photosensitivity and photolysis efficiency, effect of improving curing rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0112] Synthesis of 2-methoxy-4-(anilino)-diazobenzene (MSDS) acid generator of dodecylsulfate. While stirring, 16.0 g of sodium lauryl sulfate (Aldrich, Milwaukee, MI) in 300 ml of water was slowly added to 18.0 g of hydrogen sulfate 2-methoxy-4-(phenylamino)- Diazobenzene (Diverstec, Fort Collins, CO). The mixture was stored at 0-5°C in the dark for 5 hours. After decanting the water, the oil obtained was dissolved in 100 ml of ethyl acetate. with 50ml 5% NaHCO 3 The solution was washed and then washed with 50 ml of water. The organic layer was dried over anhydrous magnesium sulfate for 6 hours, and the solvent was removed by evacuation. As a result, 12.8 g of oil was obtained.
[0113] Proton NMR (in acetone-d 6 ): δ0.88(3H, t), 1.32(18H, m), 1.58(2H, m), 3.91(2H, t), 4.15(3H, s), 6.90-7.55(7H, m), 8.18( 1H, d) and 11.12 (1H, s).
Synthetic example 2
[0115] Synthesis of hexadecylsulfate 2-methoxy-4-(anilino)-diazobenzene (MSHDS) acid generator. Utilize 0.8g NaHCO in 25ml water 3 Neutralize 3.25 grams of 2-methoxy-4-(anilino)-diazobenzene bisulfate (Diverstec, Fort Collins, CO) in 50 ml of water and label the container A. Dissolve 3.45 g of sodium cetyl sulfate (TCI America, Portland, OR) in 150 ml of water (50°C) and label the container B. With stirring, solution A was slowly added to solution B and a precipitate formed after mixing was complete. The reaction mixture was stored at 0-5°C in the dark for 12 hours. The solid was collected by filtration and dried under vacuum. The yield was 5.4 g.
[0116] Proton NMR (in acetone-d 6 ): δ0.87(3H, t), 1.31(26H, m), 1.58(2H, m), 3.90(2H, t), 4.15(3H, s), 6.90-7.60(7H, m), 8.19( 1H, d) and 11.10 (1H, s).
Synthetic example 3
[0118] Synthesis of vinyl benzyl thiosulfate 2-methoxy-4-(anilino)-diazobenzene (MSVBTS) acid generator. While stirring, slowly add 25 ml of 5% hydrogen sulfate 2-methoxy-4 -(anilino)-diazobenzene (Diverstec, Fort Collins, CO). The mixture was stored at 0-5°C in the dark for 24 hours. After decanting the water, the oil obtained was washed three times with 10 ml of water. As a result, 1.13 g of oil was obtained, which was then dissolved in 99 g of 1-methoxy-2-propanol for use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com