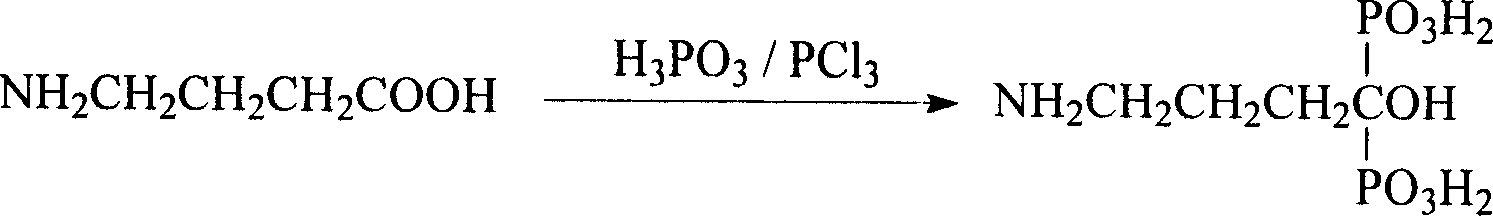Method for preparing Alendronic acid
A technology of alendronic acid and phosphorous acid is applied in the field of preparation of alendronic acid, which can solve the problems of uneconomical, unsuitable for industrial production, out of control of self-exothermic punching, etc., and achieves improved safety and good industrial application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add γ-amino acid and phosphorous acid with a molar ratio of 1:1.2 to cyclohexane that is 5 times the weight of γ-amino acid, under stirring, heat the water bath to reflux, keep warm, and when the solution becomes translucent gel, start to drop Phosphorus trichloride corresponding to 1.2 equivalents of γ-amino acid was added. Stirring with heating was continued for 4 hours. Heating was stopped, cooled to room temperature, and the solvent was removed by filtration; then an appropriate amount of distilled water was added to dissolve the jelly, and heating was continued under reflux for 1 hour. Use activated carbon to decolorize, filter to obtain a clear and colorless solution, concentrate under reduced pressure, and after cooling, add the concentrated solution dropwise to an appropriate amount of hot methanol (50°C) with stirring to produce a white precipitate, which is separated by filtration after cooling to obtain alendronic acid White crystalline powder, m.p.229°C, yi...
Embodiment 2
[0019] Add γ-amino acid and phosphorous acid with a molar ratio of 1:1.4 to benzene that is 5 times the weight of γ-amino acid, under stirring, heat in a water bath to reflux, keep warm, and after the solution is translucent gelatinous, start to drop an equivalent amount of Phosphorus trichloride in 1.2 equivalents of γ-amino acids. Stirring with heating was continued for 3.5 hours. Heating was stopped, cooled to room temperature, and the solvent was removed by filtration; then an appropriate amount of distilled water was added to dissolve the jelly, and heating was continued under reflux for 1 hour. Use activated carbon to decolorize, filter to obtain a clear and colorless solution, concentrate under reduced pressure, and after cooling, add the concentrated solution dropwise to an appropriate amount of hot methanol (50°C) with stirring to produce a white precipitate, which is separated by filtration after cooling to obtain alendronic acid White crystalline powder, m.p.229°C,...
Embodiment 3
[0021] Add γ-amino acid and phosphorous acid with a molar ratio of 1:1.3 to n-hexane that is 5 times the weight of γ-amino acid, under stirring, heat to reflux in a water bath, keep warm, and start adding dropwise after the solution becomes translucent gel Phosphorus trichloride equivalent to 1.2 equivalents of γ-amino acids. Stirring with heating was continued for 8 hours. Heating was stopped, cooled to room temperature, and the solvent was removed by filtration; then an appropriate amount of distilled water was added to dissolve the jelly, and heating was continued under reflux for 1 hour. Use activated carbon to decolorize, filter to obtain a clear and colorless solution, concentrate under reduced pressure, and after cooling, add the concentrated solution dropwise to an appropriate amount of hot methanol (50°C) with stirring to produce a white precipitate, which is separated by filtration after cooling to obtain alendronic acid White crystalline powder, m.p.229°C, yield 57...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com