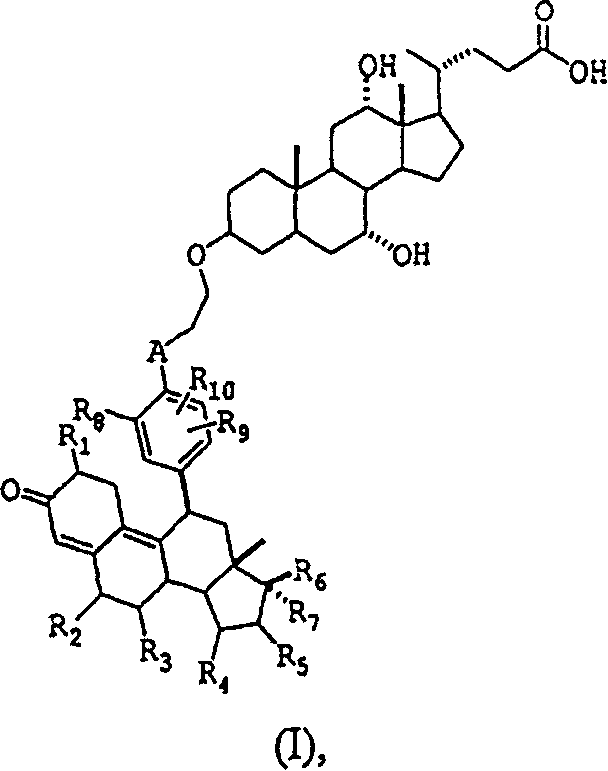
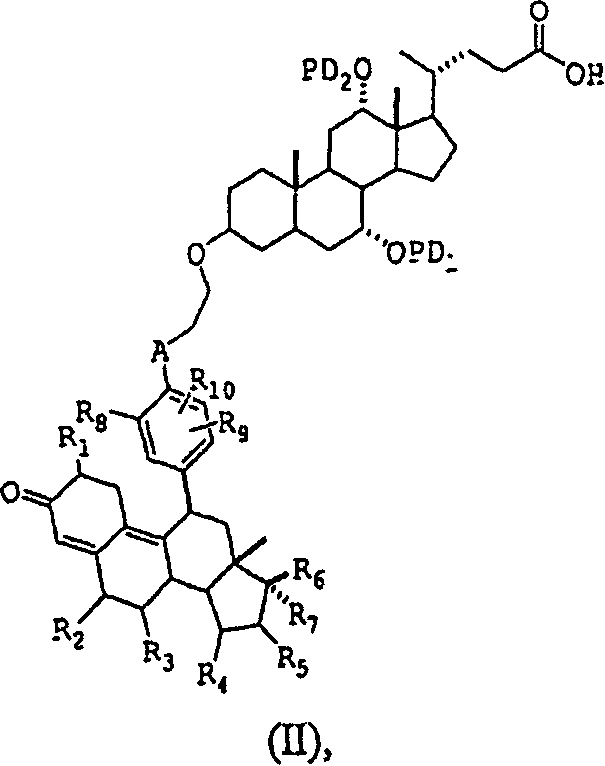
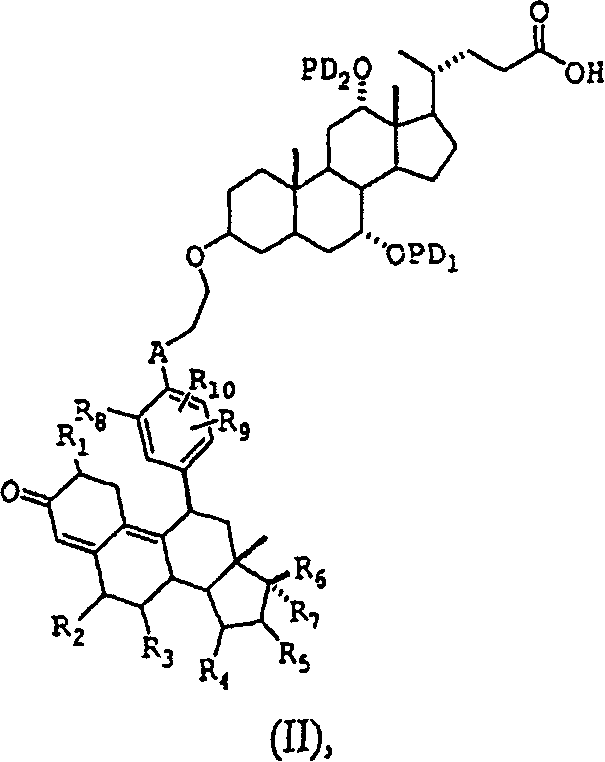
Glucocorticoid receptor ligands for the treatment of metabolic disorders
An alkyl and alkynyl technology, applied in the field of glucocorticoid receptor ligands for the treatment of metabolic disorders, can solve problems such as glucocorticoid deficiency, excess glucose, and death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0090] Scheme 1. Preparation of Activated Bile Acid Intermediates
[0091]
[0092] Scheme 2. Preparation of intermediates of glucocorticoid antagonists
[0093]
[0094] Scenario 3. Combination of Fragments
[0095]
[0096] Compounds of the invention can be prepared according to the methods described in Schemes 1-3. One approach to introduce potential linker groups on cholic acid intermediates is described in Scheme 1. Methyl cholate is selectively activated at the 3-position, for example, by treatment with a sulfonyl halide or the like. The activated 3-alcohol is replaced with ethylene glycol, and the resulting alcohol is activated as a leaving group, for example by conversion to chloride, sulfonate, etc., to obtain intermediate Z.
[0097] In Scheme 2, intermediates A and B are prepared from well-known ketone-ketals in the following steps. Addition of organometallic reagents such as propynyl-magnesium bromide etc. to C-17 ketones yields the corresponding beta ...
Embodiment 1
[0129] (3β, 5β, 7α, 12α)-7,12-dihydroxy-3-{2-[{4-[(11β, 17α)-17-hydroxy-3-oxo-17-1-prop-1-yne base-4,9- Estradien-11-yl]phenyl}(methyl)amino]ethoxy}-24-cholanic acid
[0130]
Embodiment 1A
[0132] (3α, 5β, 7α, 12α)-7,12-dihydroxy-3-(methylsulfonyloxy)-24-cholanoic acid methyl ester
[0133] To a solution of methyl cholate (25 g, 59.2 mmol) in pyridine (75 mL) was added methanesulfonyl chloride (5.04 mL, 65.1 mmol) dropwise with stirring at 0°C during 30 minutes. The reaction was warmed to room temperature and stirred for 6 hours. The reaction mixture was poured into a mixture of EtOAc (200 mL), 1N HCl (200 mL) and ice. The layers were separated, and the organic layer was washed with 1N HCl (2×50 mL), dried (Na 2 SO 4 ) and concentrated to obtain light yellow oil. The crude material was passed through a silica plug eluting with 50% EtOAc / Hexanes to afford 24.5 g (83%) of the title compound as a light yellow oil which formed a white sticky mass when placed under high vacuum pump bubbles.
[0134]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com