Oxo synthesis catalyst and its prepn process and application in preparing acetic acid and acetic anhydride
An oxo synthesis and catalyst technology, applied in the field of oxo catalysts, can solve the problems of increased corrosiveness of reaction medium, loss of activity, limitation of production efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Weigh out sodium pyridine-3-carboxylate, NdCl 3 0.01 mol each, dissolved in an aqueous solution containing 1 mol of methanol, in which 0.5 mol of water, stirred and reacted at 70°C for 1 h to obtain a solution of pyridine-3-carboxylic acid sodium salt neodymium complex.
[0038] In the same way, sodium pyridine-3-formate, sodium pyridine-2-formate, sodium pyridine-4-formate, sodium pyridine-2-acetate, sodium pyridine-3-acetate, sodium pyridine 4-acetate and rare earth metal salts were reacted to obtain the above-mentioned Solution of complexes of pyridine carboxylates with lanthanum, cerium and neodymium.
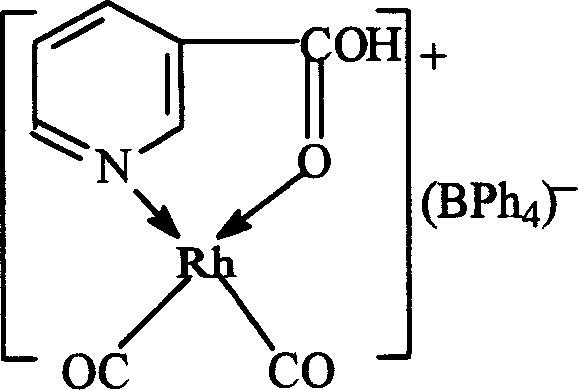
[0039] Weigh 0.01mol of pyridine-3-carboxylic acid and 0.005mol of [Rh(CO) 2 Cl] 2 Dissolve it in 1.5 mol of methanol, stir and react at 70°C for 1 h to obtain a monodentate coordinated rhodium pyridine-3-carboxylate complex solution. Then add methanol-water mixed solution containing 0.005 mol of pyridine-3-formic acid sodium neodymium complex (the molar ratio of ...
Embodiment 2
[0042] Take by weighing 0.3g of pyridine-2-formic acid rhodium-neodymium positive and negative ion type bimetallic catalyst made in Example 1, methanol 1.24mol, acetic acid 0.87mol, methyl iodide 0.24mol, join in the 250ml zirconium reactor, pass into CO Afterwards, the temperature was raised to 170°C, the constant pressure was 4.0MPa, the stirring speed was 500 rpm, and the reaction time was 30min. The conversion rate of methanol is 100%, 0.1 mol of methyl acetate is obtained, the increment of acetic acid is 1.11 mol, and the space-time yield of acetic acid is 19.14 mol / L·h.
Embodiment 3
[0044] Take by weighing made pyridine-3-formic acid rhodium-cerium positive and negative ion type bimetallic catalyst 0.3g in embodiment 1, methyl alcohol 1.24mol, acetic acid 0.87mol, methyl iodide 0.24mol, join in the 250ml zirconium reactor, pass into CO Afterwards, the temperature was raised to 180°C, the constant pressure was 4.0MPa, the stirring speed was 500 rpm, and the reaction time was 20min. The conversion rate of methanol was 100%, and a trace amount of methyl acetate was obtained, the increment of acetic acid was 1.2 mol, and the space-time yield of acetic acid was 31.3 mol / L·h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com