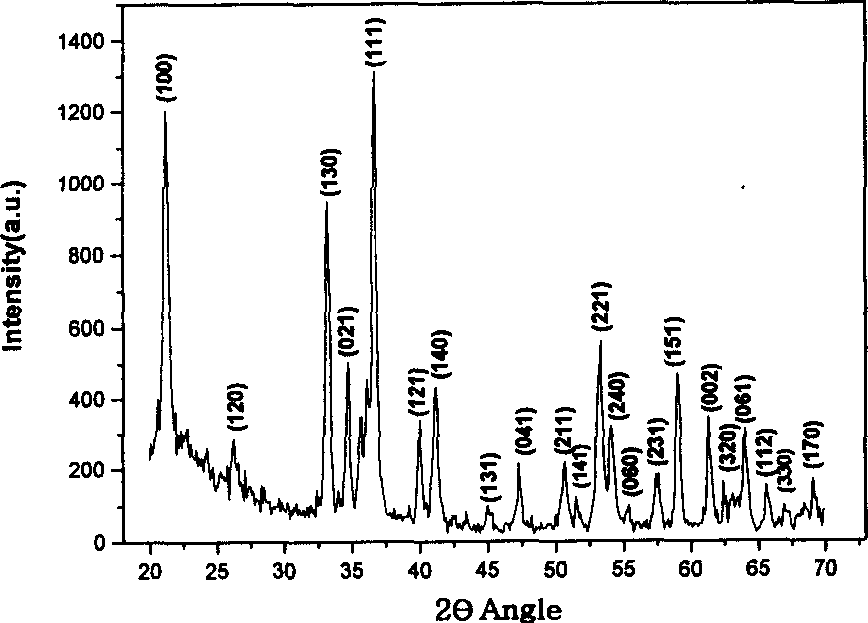
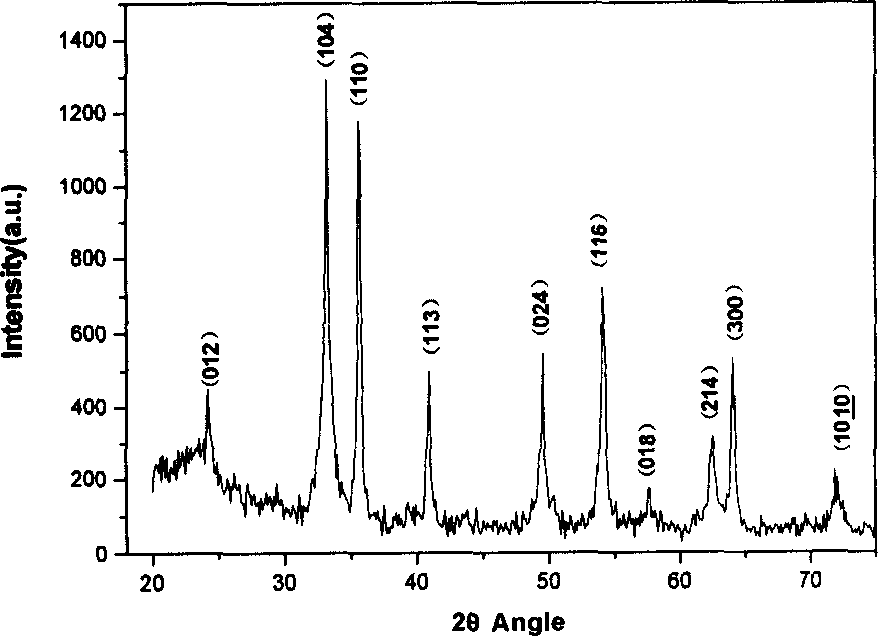
Method for producing nano material of alpha-Fe00H and alpha Fe2O3 in one dimension
A nanomaterial and production method technology, applied in the directions of iron oxide/iron hydroxide, iron oxide, etc., can solve the problems of difficulty in realizing large-scale industrial production, complicated operation steps, high cost, and achieve high yield, low cost, simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Weigh 0.0005mol of ferrous sulfate and 0.001mol of anhydrous sodium acetate and add 20ml of water to dissolve, electromagnetically stir in the air for 20min, transfer to a 20ml stainless steel reaction kettle, conduct a hydrothermal reaction at 110°C for 8 hours, and then cool the completely reacted product to room temperature, and finally washed with deionized water and vacuum-dried to obtain the product α-FeOOH nanorods. The obtained α-FeOOH nanorods were calcined in a muffle furnace at 240 °C for 2 hours to obtain α-Fe 2 o 3 Nano stave.
Embodiment 2
[0021] Weigh 0.0005mol ferrous chloride and 0.001mol anhydrous sodium acetate, add 20ml water to dissolve, electromagnetically stir in the air for 5min, transfer to a 20ml stainless steel reaction kettle, react hydrothermally at 80°C for 5 hours, and then cool the completely reacted product to room temperature, and finally washed with deionized water and vacuum-dried to obtain the product α-FeOOH nanorods. The obtained α-FeOOH nanorods were calcined in a muffle furnace at 300 °C for 1.5 hours to obtain α-Fe 2 o 3 Nano stave.
Embodiment 3
[0023] Weigh 0.001mol of ferrous sulfate and 0.002mol of anhydrous potassium acetate and add 20ml of water to dissolve, electromagnetically stir in the air for 30min, transfer to a 20ml stainless steel reaction kettle, conduct a hydrothermal reaction at 60°C for 12 hours, then cool the completely reacted product to room temperature, and finally washed with deionized water and vacuum-dried to obtain α-FeOOH nanorods. The obtained α-FeOOH nanorods were calcined in a muffle furnace at 360 °C for 1.5 hours to obtain α-Fe 2 o 3 Nano stave.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com