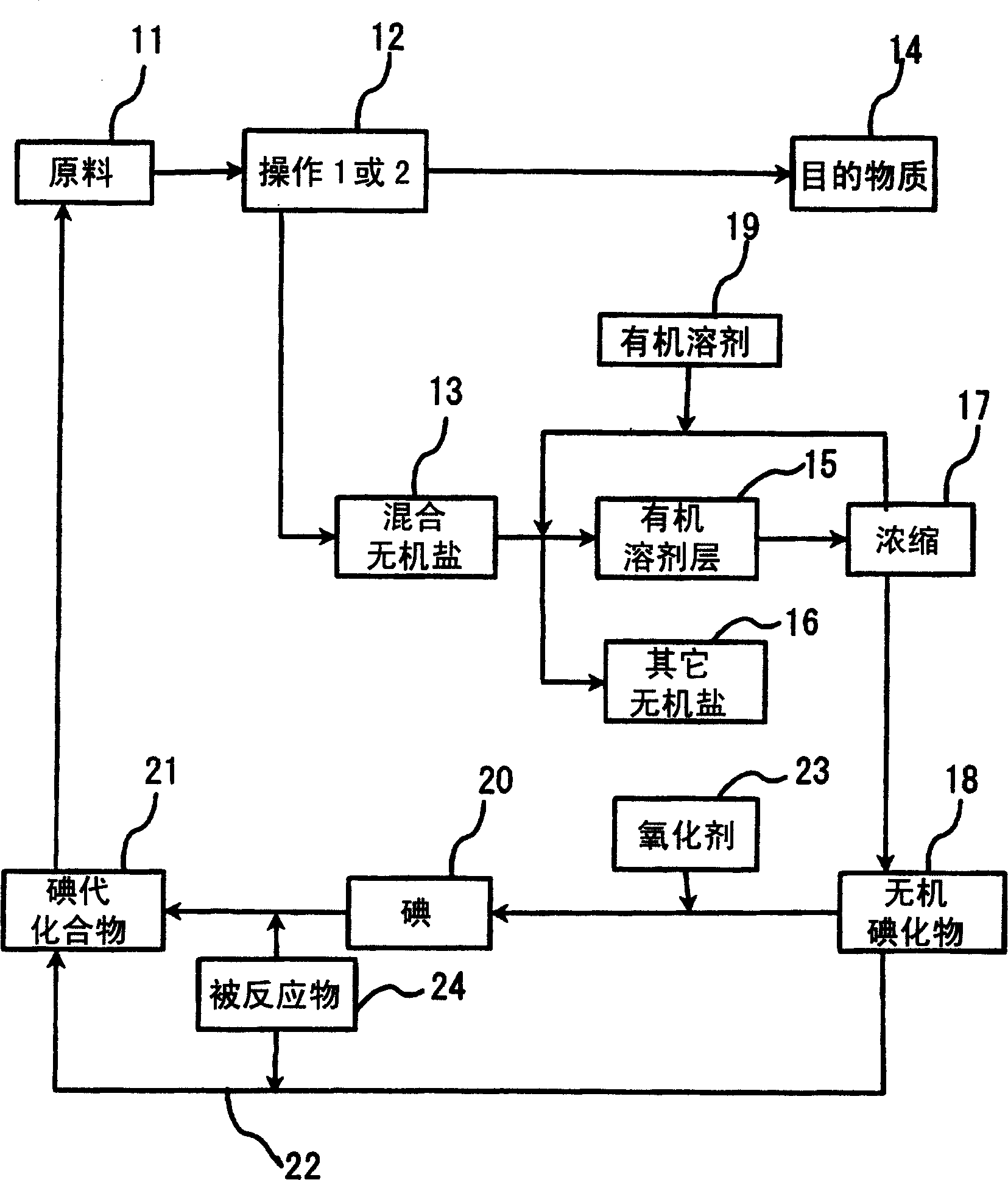
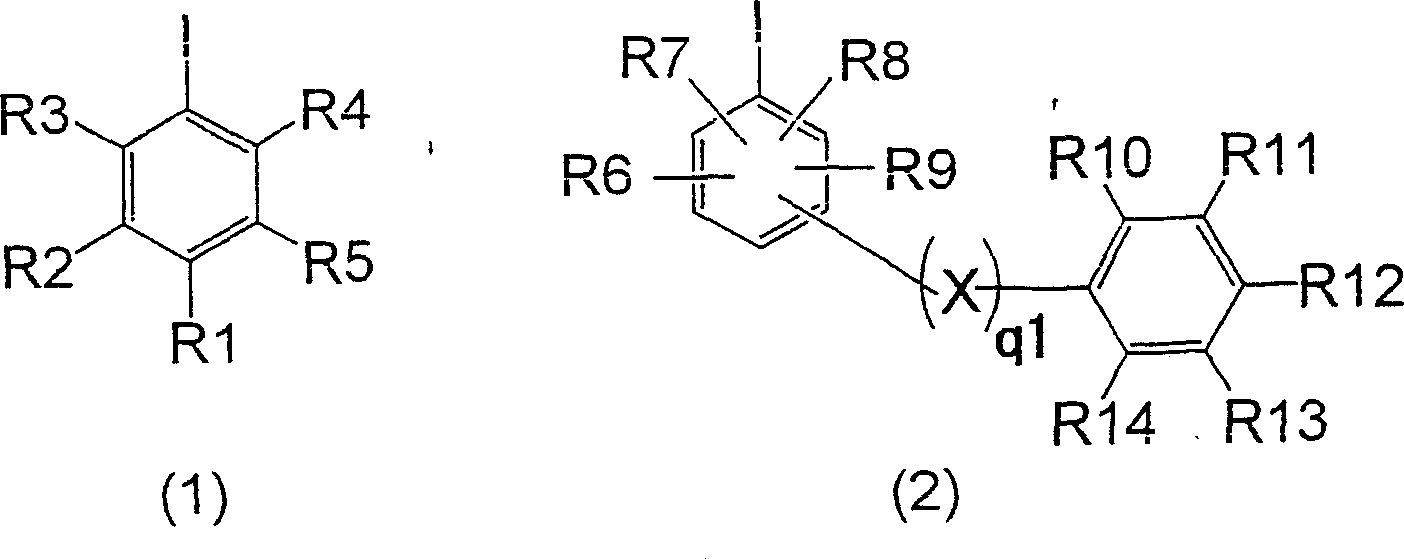
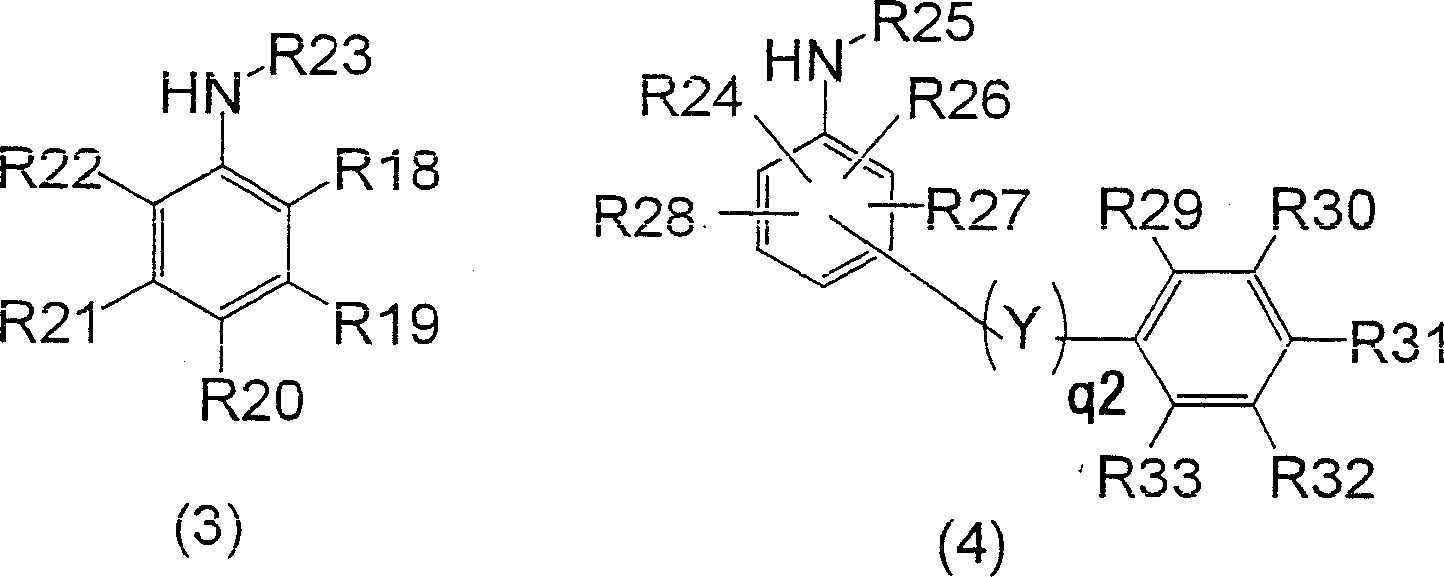
Reclaiming method of inorganic iodide
A recovery method, iodide technology, applied in the direction of iodide preparation, alkali metal iodide, chemical instruments and methods, etc., to achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Put 104.4g (616.9mmol) of diphenylamine, 62.7g (154.4mmol) of 4,4'-diiodobiphenyl, 42.1g (300mmol) of potassium carbonate, and 0.3g (1.2mmol) of copper sulfate pentahydrate into a four-necked flask , the reaction was carried out at an internal temperature of 235-240°C for 3 hours. After the reaction, the internal temperature was cooled to 100°C, 200 ml of toluene and 57.4 ml of water were added, stirred for 10 minutes, and then left to stand for 10 minutes to separate the organic layer and the water layer. After the two layers were separated, the organic layer was concentrated under reduced pressure to remove the solvent, 245ml of acetone was added, filtered after crystallization, and dried to obtain 67.9g of N,N,N',N'-tetraphenylbenzidine (collected rate 90.0%). The water layer was taken out, and 126 g was obtained as a mixed inorganic salt aqueous solution containing potassium iodide. Then, 252 ml of acetone was added to the obtained water layer, shaken and mixed fo...
Embodiment 2-21
[0107] In Example 1, it carried out by the same method as Example 1 except changing the organic solvent used and its usage-amount. The results are shown in Table 1.
[0108] Example
Embodiment 22
[0110] Put 104.4g (616.9mmol) of diphenylamine, 62.7g (154.4mmol) of 4,4'-diiodobiphenyl, 32.3g (300mmol) of sodium carbonate, and 0.3g (1.2mmol) of copper sulfate pentahydrate into a four-necked flask , the reaction was carried out at an internal temperature of 235-240°C for 5 hours. After the reaction, the internal temperature was cooled to 100°C, 200ml of toluene and 95ml of water were added, stirred for 10 minutes, and left to stand at an internal temperature of 60°C for 10 minutes to separate the organic layer (upper layer) and the aqueous layer (lower layer). The organic layer was concentrated under reduced pressure to remove the solvent, and 245 ml of acetone was added for crystallization. The crystals were separated by filtration and dried to obtain 66.8 g of N,N,N',N'-tetraphenylbenzidine (88.5% yield). On the other hand, the liquid-separated aqueous layer was a mixed inorganic salt solution containing sodium iodide, and its mass was 155 g.
[0111] Then, the above-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com