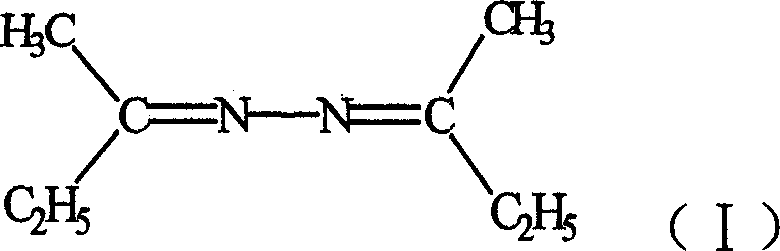
Synthesis process of methyl ethyl ketone azine
A technology for the synthesis of methyl ethyl ketone and its synthesis method, which is applied in the field of synthesis of methyl ethyl ketazine, can solve the problems of reduced yield and difficult product separation, and achieve increased yield, high product yield, and good dissolution sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Synthesis of methyl ethyl ketazine in 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid
[0046] Add cyanoacetamide (6g, 71.4mmol), 1-butyl-3-methylimidazolium tetrafluoroborate (20ml), sodium hexametaphosphate ( 0.6g, 1.0mmol), acetonitrile (20ml, 586mmol), 28% (mass content) of ammonia (50ml, 588mmol), butanone (54ml, 588mmol), dropwise 30% (mass content) of hydrogen peroxide (20ml , 196mol), stirred at 40°C for 8 hours, distilled the reaction solution, steamed off ammonia, butanone, acetonitrile, 2-butanol, water, and extracted the remaining liquid with dichloromethane to recover the ionic liquid to obtain 16.05g of the product, the yield 58.6% (calculated as hydrogen peroxide).
Embodiment 2
[0047] Example 2 Synthesis of methyl ethyl ketazine in 1-benzyl-3-methylimidazolium tetrafluoroborate ionic liquid
[0048] Add cyanoacetamide (3g, 35.7mmol), 1-benzyl-3-methylimidazolium tetrafluoroborate (10ml), sodium hexametaphosphate ( 0.3g, 0.5mmol), acetonitrile (10ml, 293mmol), 28% (mass content) of ammonia (25ml, 294mmol), methyl ethyl ketone (27ml, 294mmol), dropwise 30% (mass content) of hydrogen peroxide (10ml , 98mmol), stirred at 50°C for 8 hours, distilled the reaction solution, steamed off ammonia, butanone, acetonitrile, 2-butanol, water, and extracted the remaining liquid with dichloromethane to recover the ionic liquid to obtain 7.74g of the product, the yield 56.5% (calculated as hydrogen peroxide).
Embodiment 3
[0049] Example 3 Synthesis of methyl ethyl ketazine in 1-butyl-3-methylimidazole acetate ionic liquid
[0050] Add cyanoacetamide (3g, 35.7mmol), 1-butyl-3-methylimidazole acetate (10ml), sodium hexametaphosphate (0.3g , 0.5mmol), acetonitrile (10ml, 293mmol), 28% (mass content) ammonia (25ml, 294mmol), butanone (27ml, 294mmol), hydrogen peroxide (10ml, 98mmol) was added dropwise, and stirred at 45°C for 6 Hours, the reaction solution was distilled to remove ammonia, butanone, acetonitrile, 2-butanol, and water, and the remaining liquid was extracted with toluene to recover the ionic liquid to obtain 12.03 g of the product with a yield of 87.8% (based on hydrogen peroxide).
[0051] The ionic liquid in the reaction system can continue to be recycled.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com