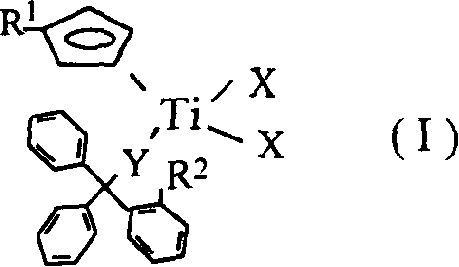Titanium catalyst containing mixed single-cyclopentadiene and monodentate great-steric hindrance ligand and its prepn and application
A technology of mixed ligands and titanium catalysts, applied in the homopolymerization or copolymerization of α-olefins, monodentate large sterically hindered non-hydrocene mixed ligand titanium compounds and its preparation, monocene field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The preparation method of the present invention for olefin polymerization or copolymerization monocene, monodentate large sterically hindered non-ocene mixed ligand titanium catalyst comprises steps as follows:
[0023] Under the protection of an inert gas, dissolve the substituted titanium trichloride and the substituted triphenylmethanol in an organic solvent, and stir the reaction in the presence of pyridine, triethylamine or n-butyllithium; the reaction temperature is -80-100 ℃, the reaction time is 1-24 hours, preferably 15-16 hours, filter after the reaction, drain the clear liquid, recrystallize with an organic solvent, and wash the crystalline solid with an organic solvent several times to obtain an analytically pure catalyst. The molar ratio of titanium trichloride to substituted triphenylmethanol is 1:1-3, preferably 1:1-1.2.
[0024] The organic solvent in the preparation method of the present invention is tetrahydrofuran, ether, n-hexane, toluene, benzene, c...
Embodiment 1
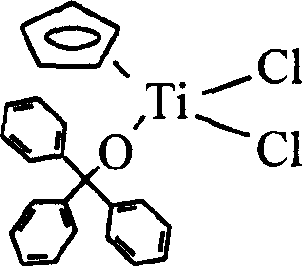
[0037] Add 1.25g (5.7mmol) CpTiCl to a 250mL Schlenk bottle 3 , add 100ml ether to dissolve it, and form a clear yellow solution. At room temperature, slowly add 1.48g (5.7mmol) Ph 3 The mixed solution of COH and 0.45g (5.7mmol) pyridine in 40ml of diethyl ether was added dropwise and stirred overnight to generate a large amount of white flocculent precipitates. After filtration, the clear liquid was sucked dry, and recrystallized with toluene / petroleum ether to obtain 11.98 g of yellow-green crystals with a yield of 78%. The molecular structural formula is as follows:
[0038]
[0039] Molecular formula: C 24 h 20 OTiCl 2
[0040] 1 H NMR (CDCl 3 , δ, ppm) data: 7.41-7.27 (m, 15H, Ph), 6.32 (s, 5H, C 5 h 5 )
[0041] M.S.: (442, M + ).
[0042] Infrared data (cm -1 ): 3109(w), 3089(w), 3059(w), 3026(w), 1597(w), 1491(m), 1445(s),
[0043] 1432(w), 3(s), 1017(s), 1000(s), 854(m), 758(s), 703(s).
[0044] Elemental Analysis Calculated: C: 65.03 H: 4...
Embodiment 2
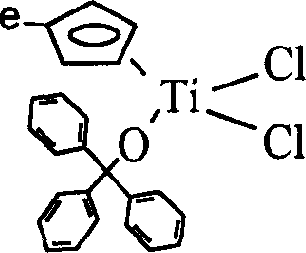
[0047] Add 0.88g (3.9mmol) Ph 3 COH and 150ml ether, add 2.1ml dropwise at -70C n BuLi (3.9mmol, 1.8mol L -1 ), after the dropwise addition was completed, it was warmed up to room temperature and continued to stir for 2 hours. Add 0.915g (3.9mmol) MeCpTiCl 3 , a LiCl precipitate was formed, stirred overnight; filtered, the clear liquid was drained, and recrystallized with toluene / petroleum ether to obtain 1.49 g of yellow-green crystal C2 with a yield of 83%. The molecular structural formula is as follows:
[0048]
[0049] Molecular formula: C 25 h 22 OTiCl 2
[0050] 1 H NMR (CDCl 3 , δ, ppm) data: 7.42-7.31 (m, 15H, Ph), 6.13 (t, J=2.70Hz, 2H, C 5 h 4 ),
[0051] 5.96(t, J=2.70Hz, 2H, C 5 h 4 ), 2.33 (s, 3H, CH 3 ).
[0052] M.S.: (456, M + ).
[0053] Infrared data (cm -1 ): 3023(w), 2924(w), 1596(w), 1492(m), 1446(m), 1375(w),
[0054] 1215(w), 1155(w), 1027(s), 1001(m), 900(w), 826(m).
[0055] Elemental Analysis Calculated: C: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com