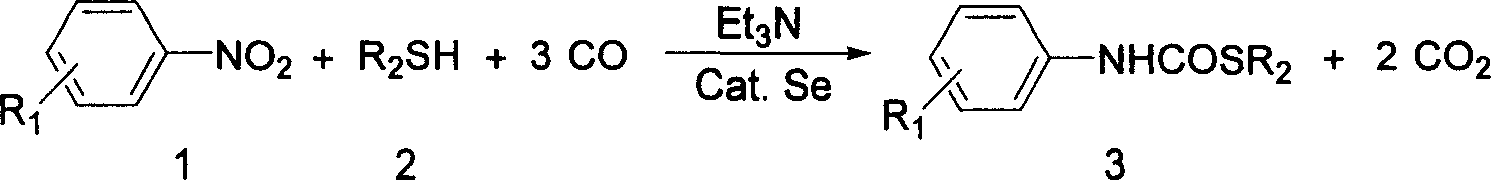Process for synthesizing thiocarbamate
A technology for synthesizing thiocarbamate and a new method, applied in the field of synthesizing thiocarbamate by catalytic reduction carbonylation reaction, can solve the problems of low atom economy, large amount of solvent, many operation steps, etc. The effect of high economy, stable product quality and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 100mL stainless steel autoclave, add nitrobenzene (10mmol), Se (0.5mmol), propanethiol (10mmol), Et 3 N (5mmol), after replacing three times with CO, raise the pressure of CO to 0.8MPa, put it into an oil bath that has risen to 50°C, stir and react for 10 hours, then cool to room temperature, open the kettle and deflate to obtain a solid crude product. Dissolve it in tetrahydrofuran, stir for 30 minutes, and filter to recover Se again. The filtrate was concentrated, purified by column chromatography, and the eluent was petroleum ether: chloroform (1:2), and the product was obtained by concentrating and removing the eluent. The product was propyl N-phenylthiocarbamate, and the yield was 83.5%. . It can also be directly recrystallized in petroleum ether to obtain colorless needle-like crystals.
Embodiment 2
[0022] The mercaptan is ethanethiol, and the consumption is 10 mmol. Other experimental methods and conditions are the same as in Example 1. The product is ethyl N-phenylthiocarbamate, and the actual yield is 80.0%.
Embodiment 3
[0024] The mercaptan is isopropyl thiol, and the consumption is 10 mmol. Other experimental methods and conditions are the same as in Example 1. The product is isopropyl N-phenylthiocarbamate, and the actual yield is 57.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com