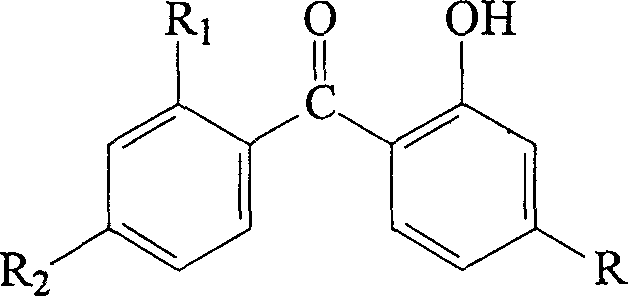
O-hydroxyl-diphenyl ketone- rare earth complex and its preparation method and uses
A technology of o-hydroxybenzophenone and rare earth complexes, applied in the direction of organic chemistry, can solve the problems of low volatility, increased difficulty in implementing modification technology, and increased cost, and achieve the effect of less blooming phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] At room temperature, dissolve 13.7g (0.06mol) of 2-hydroxy-4-methoxybenzophenone (UV-9, HMBP) in 300mL of absolute ethanol, add 60mL of 1mol·L -1 Sodium hydroxide (0.06mol) aqueous solution was stirred and mixed for reaction, then, slowly added dropwise 40mL 0.5mol L -1 Samarium Chloride (SmCl 3 , 0.02mol) aqueous solution, continued to stir and react for 1.5 hours. The precipitate generated by the reaction was aged at room temperature for 24 hours and then filtered under reduced pressure. The filter cake was washed with 1:1 ethanol aqueous solution until the filtrate was tested with acidified silver nitrate solution to detect no chloride ions, and then dried at 15°C under normal pressure to constant weight to obtain shallow Yellow powdery product. After analysis and determination, the Sm, C, and H contents of the product are 17.87%, 60.79% and 4.66% respectively, which is the same as the chemical formula Sm(MBP) 3 Consistent (the calculated values of Sm, C, and H ...
Embodiment 2
[0035] According to the synthesis method and conditions of Example 1, the SmCl 3 Sm in is replaced by Pr, Nd, Eu, Tb, Dy, Ho, Er, Tm, Yb respectively, and the composition can be obtained as RE(MBP) 3 (RE stands for Pr, Nd, Eu, Tb, Dy, Ho, Er, Tm or Yb) light yellow powder product.
Embodiment 3
[0037] At room temperature, dissolve 19.6g (0.06mol) of 2-hydroxy-4-n-E-octyloxybenzophenone (UV-531, HOBP) in 300mL of absolute ethanol, add 60mL of 1mol·L -1 Sodium hydroxide (0.06mol) aqueous solution was stirred and mixed for reaction, then, slowly added dropwise 40mL 0.5mol L -1 Samarium Chloride (SmCl 3, 0.02mol) aqueous solution, continued to stir and react for 1.5 hours. The precipitate formed by the reaction was aged at room temperature for 24 hours and then filtered under reduced pressure. The filter cake was washed with 1:1 aqueous ethanol until the filtrate was tested with acidified silver nitrate solution to detect no chloride ions, and then dried at 150°C under normal pressure to constant weight to obtain Pale yellow semi-solid product. After analysis and determination, the Sm, C, and H contents of the product are 13.31%, 67.21%, and 7.30% respectively, which is the same as the chemical formula Sm(OBP) 3 They are consistent (the calculated values of Sm, C, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com