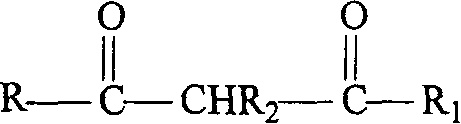Use of beta-diketone-rare earth complex as light stabilizer
A technology of rare earth complexes and light stabilizers, which is applied in the application field of β-diketone-rare earth complexes as light stabilizers, can solve the problems of low volatility, high toxicity and environmental pollution, and comprehensive technical and economic performance improvement. Satisfaction and other issues, to achieve the effect of small blooming phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] At room temperature, dissolve 9.72g (0.06mol) of benzoylacetone (HBA) in 300mL of absolute ethanol, add 60mL of 1mol L -1 Sodium hydroxide (0.06mol) aqueous solution was stirred and mixed for reaction, then, slowly added dropwise 40mL of 0.5mol L -1 Samarium Chloride (SmCl 3 , 0.02mol) aqueous solution, continued to stir and react for 1.5 hours. The precipitate formed by the reaction was aged at room temperature for 24 hours and then filtered under reduced pressure. The filter cake was washed with 1:1 aqueous ethanol until the filtrate was tested with acidified silver nitrate solution to detect no chloride ions, and then dried at 150°C under normal pressure to constant weight to obtain Light yellow powder product. After analysis and determination, the Sm, C, and H contents of the product are 22.89%, 57.18%, and 4.97% respectively, which is the same as the chemical formula Sm(BA) 3 Consistent (the calculated values of Sm, C, and H contents are 23.72%, 56.84%, and 4....
Embodiment 2
[0036] According to the synthesis method and conditions of Example 1, the SmCl 3 Sm in is replaced by Pr, Nd, Eu, Tb, Dy, Ho, Er, Tm, Yb respectively, and the composition can be obtained as RE(BA) 3 (RE stands for Pr, Nd, Eu, Tb, Dy, Ho, Er, Tm or Yb) yellowish powdery product.
Embodiment 3
[0038] At room temperature, dissolve 13.44g (0.06mol) of dibenzoylmethane (HDBM) in 300mL of absolute ethanol, add 60mL of 1mol L -1 Sodium hydroxide (0.06mol) aqueous solution was stirred and mixed for reaction, then, slowly added dropwise 40mL of 0.5mol L -1 Samarium Chloride (SmCl 3 , 0.02mol) aqueous solution, continued to stir and react for 1.5 hours. The precipitate formed by the reaction was aged at room temperature for 24 hours and then filtered under reduced pressure. The filter cake was washed with 1:1 aqueous ethanol until the filtrate was tested with acidified silver nitrate solution to detect no chloride ions, and then dried at 150°C under normal pressure to constant weight to obtain Light yellow powder product. After analysis and determination, the Sm, C, and H contents of the product are 18.49%, 65.68%, and 4.40% respectively, which is the same as the chemical formula Sm(DBM) 3 They are consistent (the calculated values of Sm, C, and H contents are 18.33%, 65...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com