Method for photochemosynthesis of vitamin D2
A technology for photochemical synthesis and vitamins, which is applied in organic chemistry and other fields, can solve problems such as troublesome post-processing, difficulty in large-scale production, and difficulty in realizing industrialization, and achieves the effect of simple production process and increased concentration of reactants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
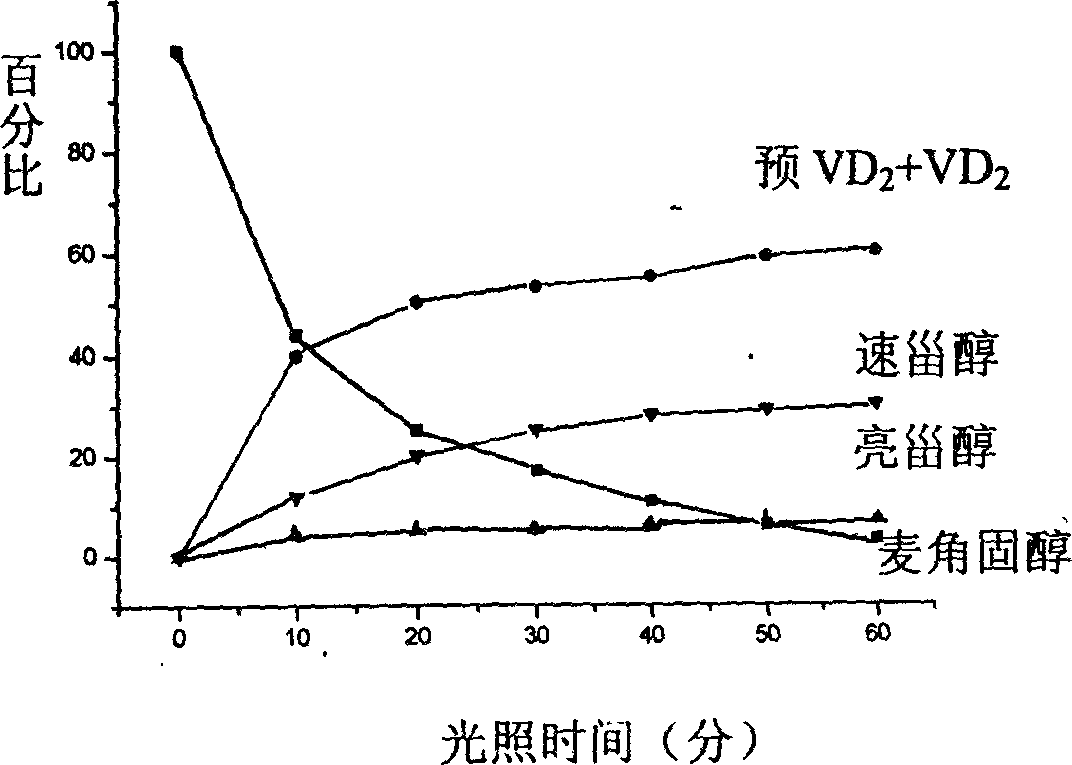
[0039] (1) Photoresponse of ergosterol
[0040] In a 500-liter batching kettle, at room temperature, 2 kg of ergosterol was dissolved in 100 liters of hexane-ethanol (2:1, V / V) mixed solvent, and 1.4 grams of 2,6-di-tert-butyl- P-methoxyphenol, stir and mix evenly, and configure the photochemical reaction solution. The reaction solution was pumped into a 4 kW high-pressure mercury lamp (product of UV-Consulting Peschl, Germany) and a large-scale photochemical reactor of internal immersion upward bubbling type with nitrogen gas. Adjust the nitrogen flow rate to make the bubbles uniform. Start the mercury lamp. The reaction solution was flowed through the photoreactor at a flow rate of 1.4 liters / minute for illumination. A cold water jacket and shading jacket are added to the reaction device to ensure that the temperature of the photochemical reaction solution does not exceed 28°C to avoid premature formation of vitamin D during the photochemical reaction 2 . The reaction l...
Embodiment 2
[0052] (1) Photoresponse of ergosterol
[0053] In a 500-liter batching kettle, dissolve 2 kilograms of ergosterol in 100 liters of petroleum ether (60-90° C.)-methanol (7:1, V / V) mixed solvent, and add 1.4 grams of 2,6-di-tert-butyl Base-p-methoxyphenol, stir and mix evenly, and configure the photochemical reaction solution. Following process and condition are with embodiment 1.
[0054] (2) reclaim ergosterol
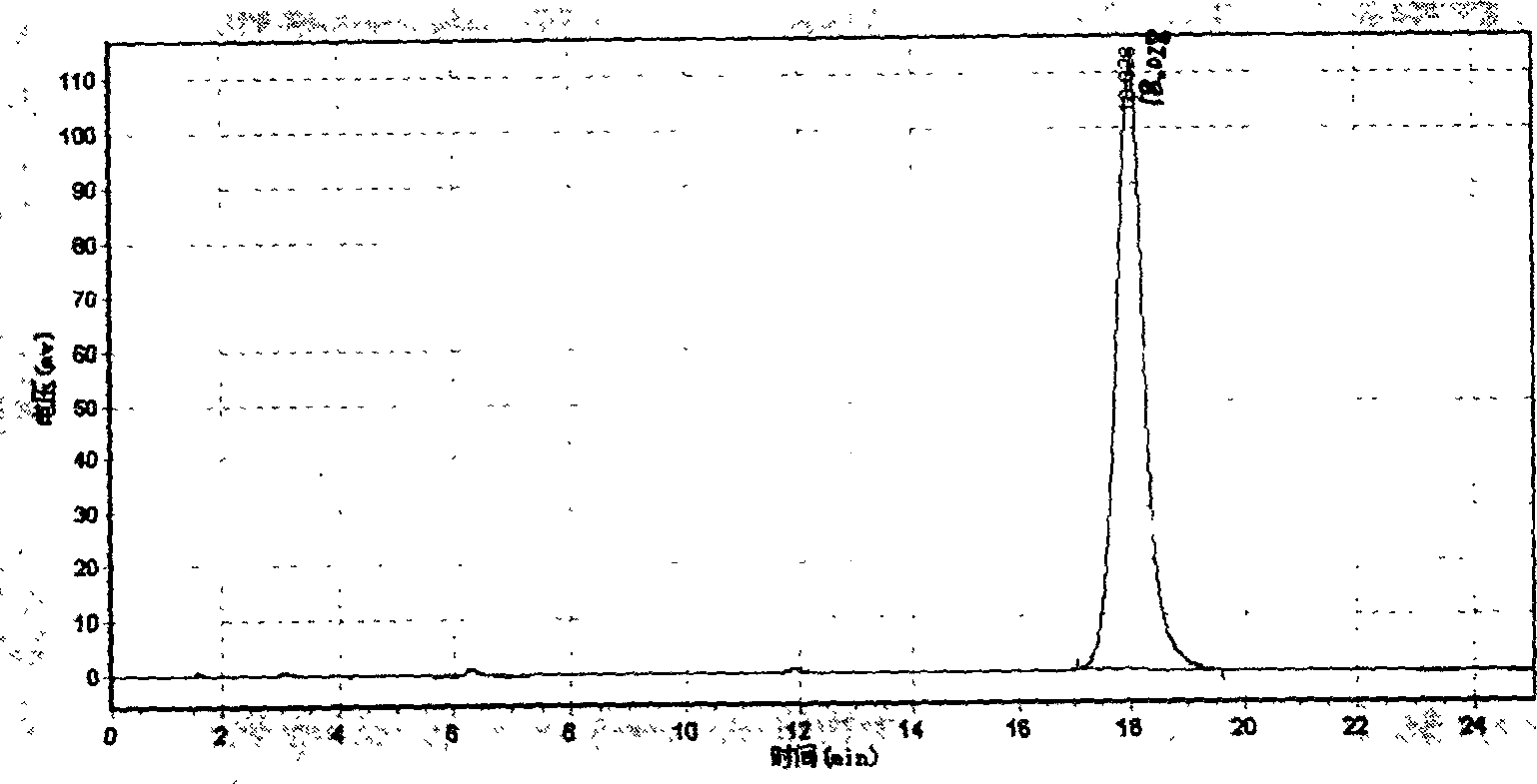
[0055] After the above photochemical reaction was completed, the reaction solution was successively pumped into the kettle of a 50-liter integrated reactor (QVF Compact Reactor), evaporated to dryness under reduced pressure, and the solvent was recovered. An additional 10 liters of methanol was added and frozen overnight at -20°C. The next day, it was quickly centrifuged by a centrifuge (Henkel HF 300) to obtain 1.31 kg of solid ergosterol (which can be used as a raw material for the next photoreaction).
[0056] (3) Thermal isomerization reaction to produce vitam...
Embodiment 3
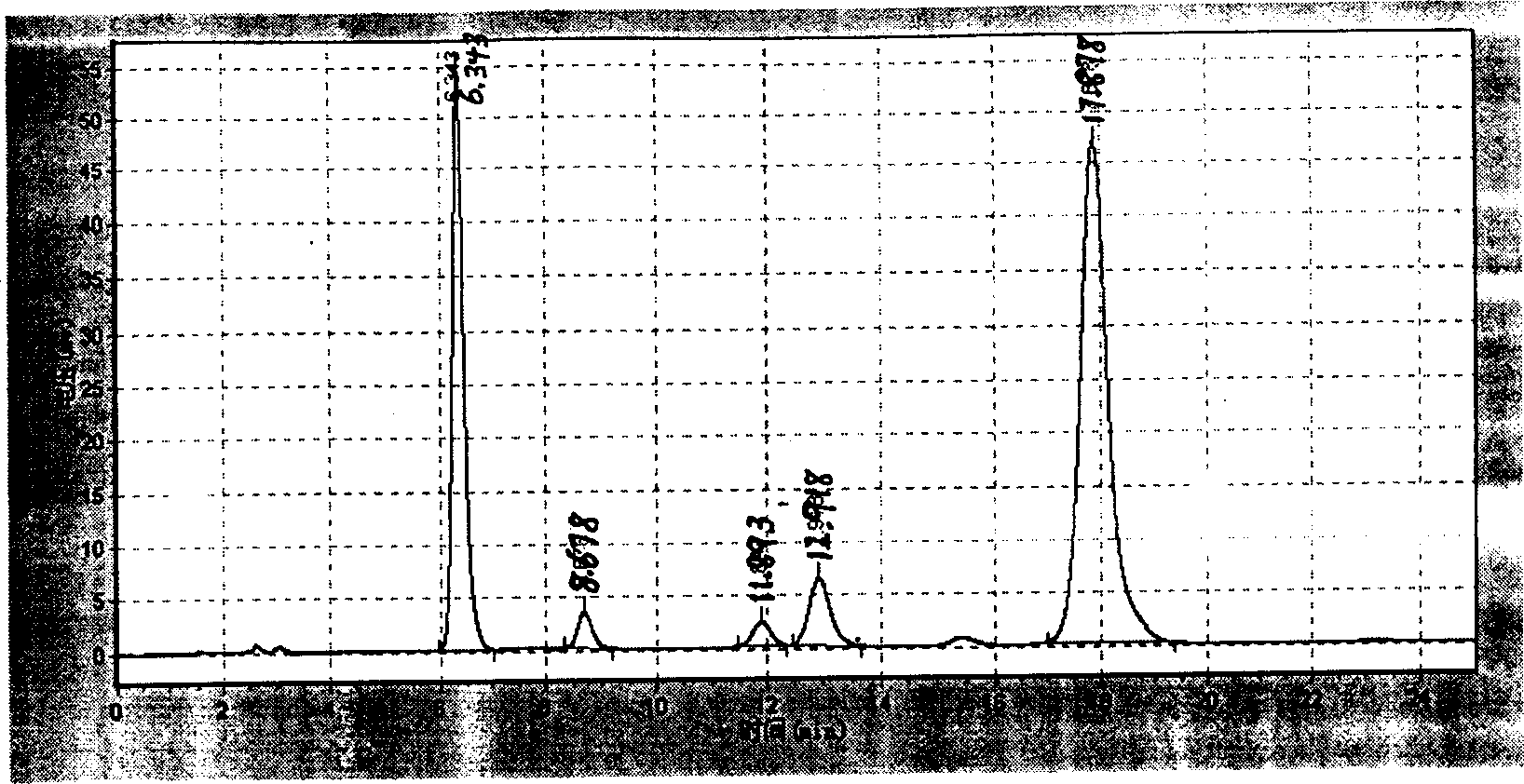
[0065] (1) Photoresponse of ergosterol
[0066] In a 500-liter batching kettle, dissolve 4 kilograms of ergosterol in 200 liters of hexane-methanol (9:1, V / V) mixed solvent, add 2.4 grams of 2,6-di-tert-butyl-p-cresol , stirred and mixed evenly, and configured into a photochemical reaction solution. The reaction solution is pumped into a 10 kW high-pressure mercury lamp (product of UV-Consulting Peschl, Germany) and nitrogen-filled large-scale photochemical reactor with internal immersion upward bubbling. Adjust the nitrogen flow rate to make the bubbles uniform. Start the mercury lamp. The reaction solution was flowed through the photoreactor at a flow rate of 2.8 liters / minute for illumination. A cold water jacket and shading jacket are added to the reaction device to ensure that the temperature of the photochemical reaction solution does not exceed 28°C to avoid premature formation of vitamin D during the photochemical reaction 2 . The reaction liquid flowed while bein...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com