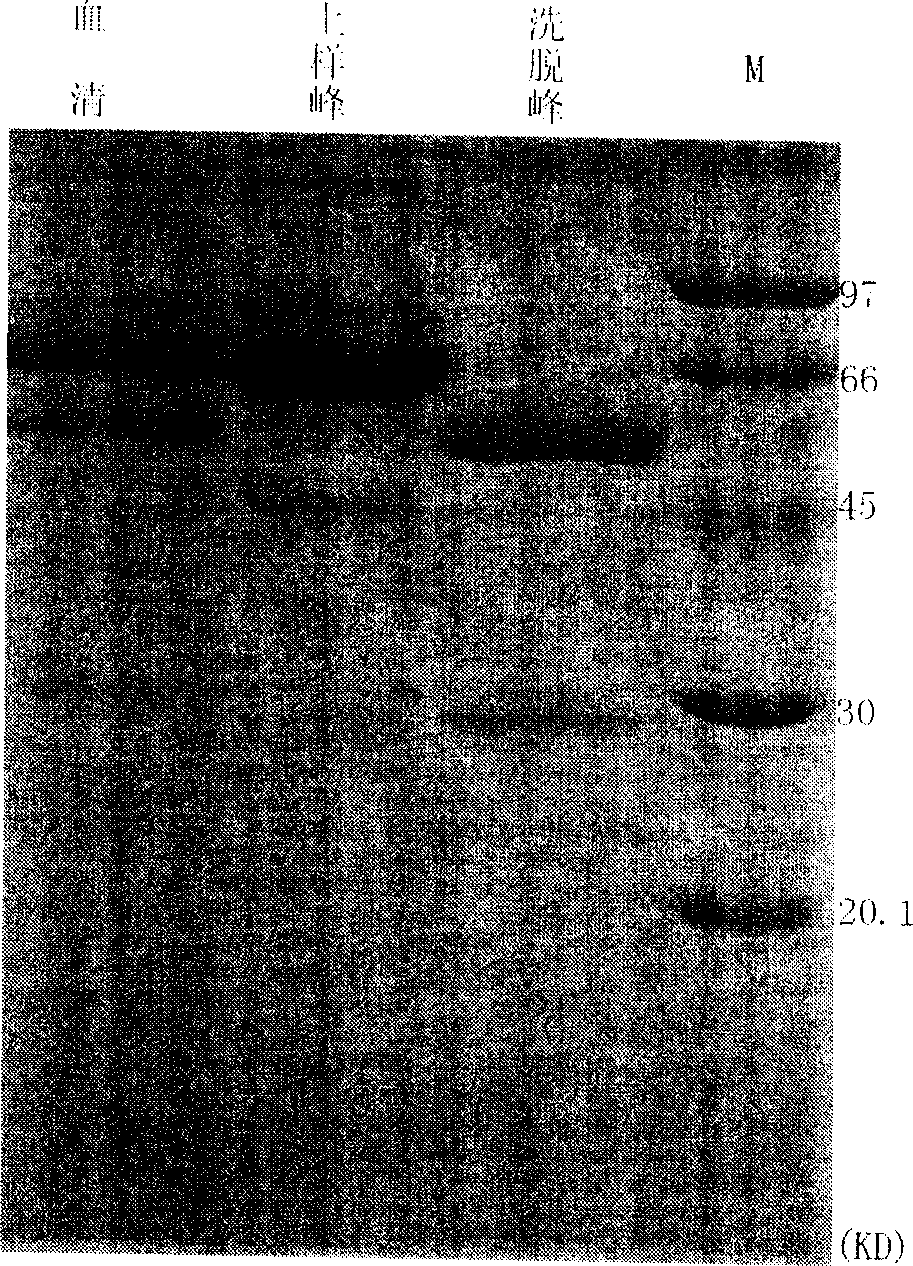Purification process and application of antigen capable of being used in diagnosis of multiple myitis/dermatomyositis
A purification method and dermatomyositis technology, applied to the antigen purification process and application field that can be used for the diagnosis of polymyositis/dermatomyositis, can solve the problems of long time consumption, high cost, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Extractable nuclear antigen extraction and manufacture of extractable nuclear antigen acetone powder
[0083] 1 Take fresh or -80°C frozen pig spleen and other animal tissues, and the frozen tissue needs to be thawed and recovered at 4°C overnight (more than 12 hours);
[0084] 2 Weigh 100g of porcine spleen and other tissues, add 200ml homogenization buffer (0.01M PBS, pH7.0, disodium edetate 0.01M, phenylmethylsulfonyl fluoride 1.5mM, dimercaptothreitol 1mM, Trypsin inhibitor 4μg / ml, sodium bisulfite 1.5mM), low-speed homogenization, 30 seconds / time, interval 2 minutes / time, ten times in total;
[0085] 3 Magnetically stir the tissue homogenate for 1-2 hours;
[0086] 4 Centrifuge at 4°C, 10,000G, for 1 hour;
[0087] 5 Discard the precipitate, take the supernatant and centrifuge at 4°C, 100,000G, for 1 hour;
[0088] 6 Discard the precipitate, put the supernatant in a beaker, and slowly add 5 times pre-cooled acetone while stirring;
[0089] 7 4°C, 10,000G, centr...
Embodiment 2
[0096] Fabrication of Immunoaffinity Columns
[0097] 1 Epoxy activation of agarose
[0098] 1) Rinse the dry agarose gel with deionized water (200ml / g) for 3 times, and drain it on the Buchner funnel;
[0099] 2) Transfer the washed gel into aqueous sodium hydroxide solution, then add epichlorohydrin, and their final concentrations in the activation system are respectively: gel 30% (v / v), epichlorohydrin 5% (v / v), sodium hydroxide 0.4M;
[0100] 3) shaking the above suspension under mild conditions, and reacting at 40°C for 2-3 hours;
[0101] 4) Transfer the activated gel into a Buchner funnel to drain, rinse the gel several times with deionized water, and set aside.
[0102] 2 Manufacture of Ligand
[0103] 1) Mix the monospecific Jo-1 antiserum with an equal amount of binding buffer (20 mM sodium phosphate buffer, pH=7.0), and filter with a 0.45 μm filter;
[0104] 2) Load the above-mentioned treated antiserum onto the rProtein A affinity column, and collect the loadi...
Embodiment 3
[0126] Purification of Jo-1 Antigen by Immunoaffinity Chromatography
[0127] 1) The extractable nuclear antigen solution is dialyzed against the binding buffer for 16-24 hours, and the solution is changed at least 5 times during the dialysis process. If it is an extractable nuclear antigen acetone powder, first use the extraction buffer to extract the extractable content of the acetone powder. nuclear antigen, redialysis;
[0128] 2) Filter the extractable nuclear antigen solution with a 0.45 μm filter,
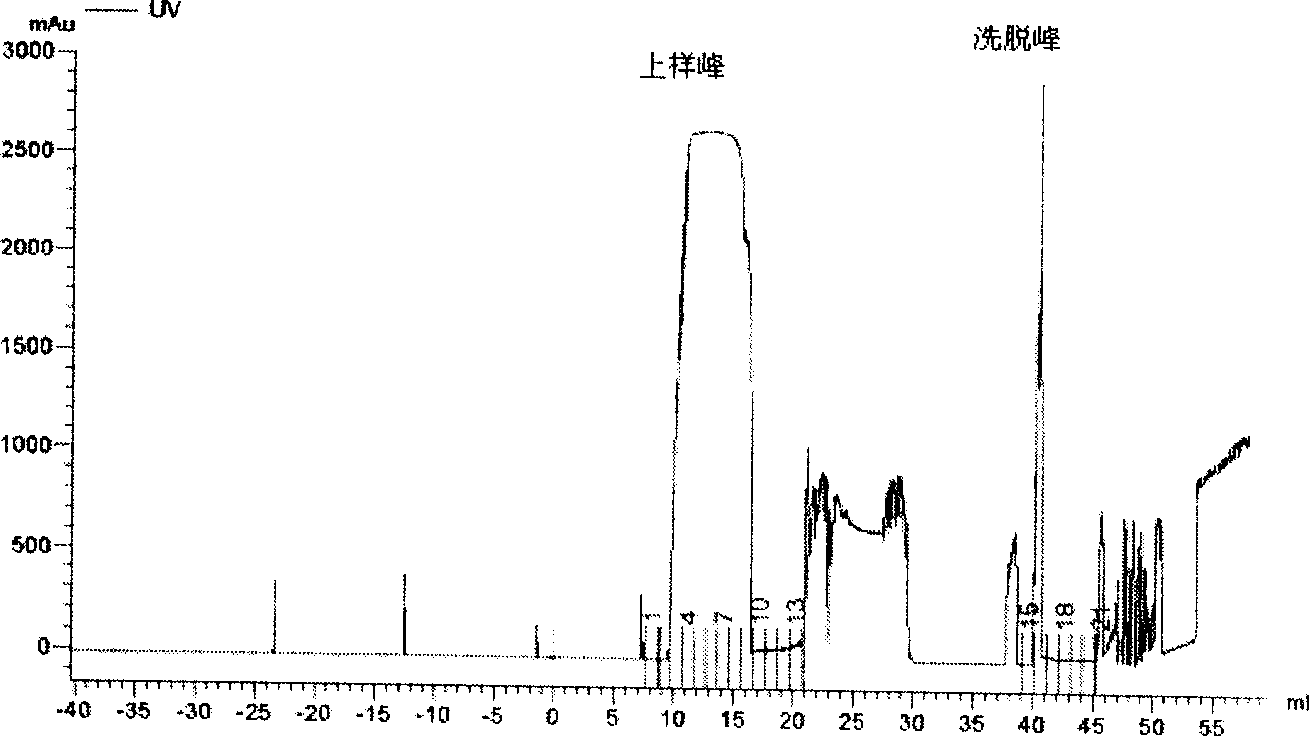
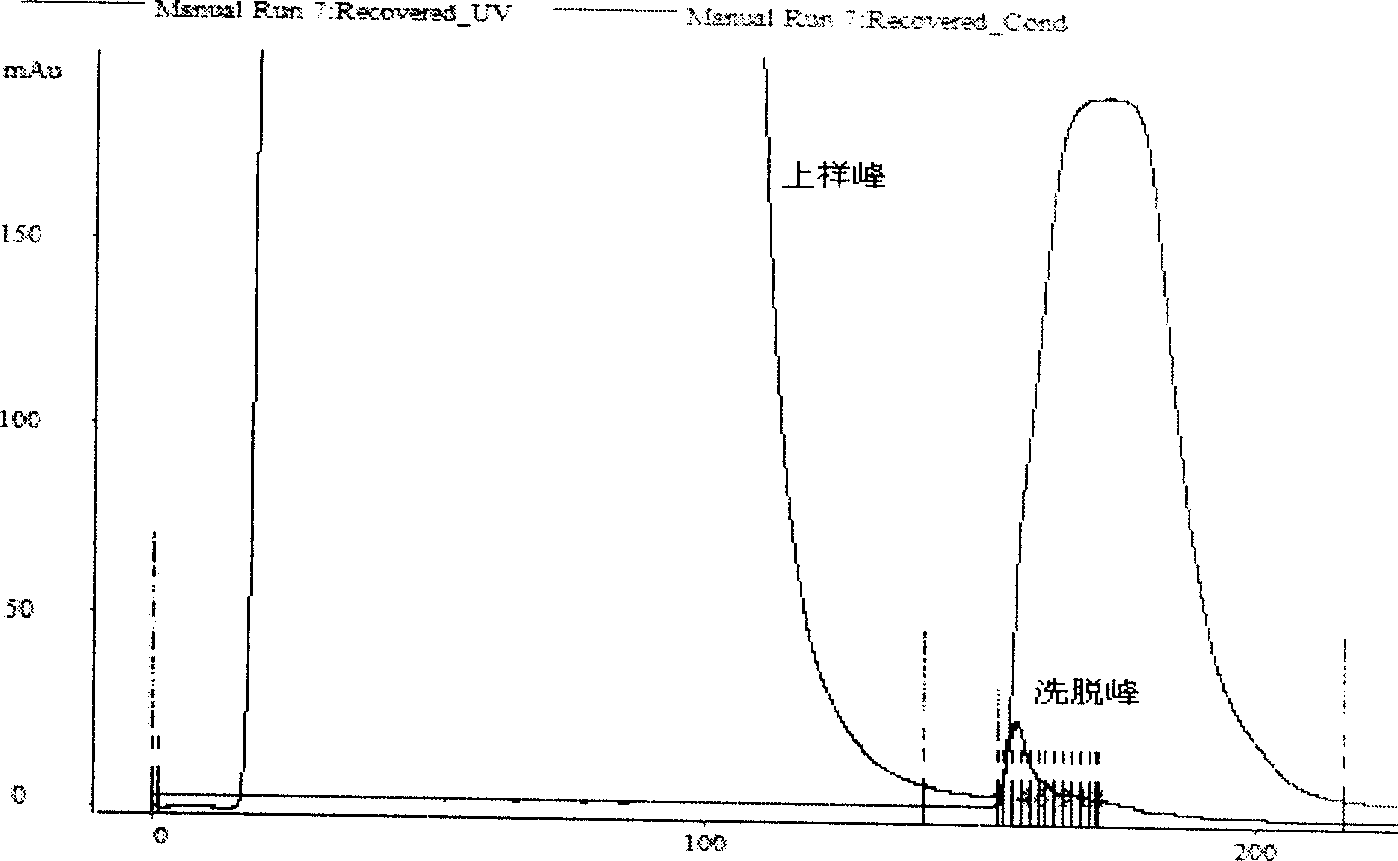
[0129] 3) Load the extractable nuclear antigen solution onto the prepared Jo-1 immunoaffinity chromatographic column, and collect the loading peaks in separate tubes;
[0130] 4) Equilibrate the affinity column with binding buffer for 5-10 column volumes;
[0131] 5) Use elution buffer (3-5M MgCl 2 ) 5-10 column volume elution, separate tubes to collect the elution peak;
[0132] 6) Equilibrate the affinity chromatography column with binding buffer for 5-10 column volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com