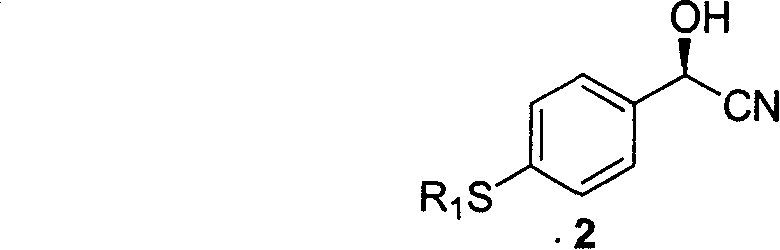Synthesis of optical active cyanhydrin compound by enzyme chemical process
A compound, cyanohydrin technology, applied in the field of enzymatic chemical synthesis, can solve problems such as waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1) Preparation of badam degreasing powder
[0046] Grind 50g of Badamia kernels in a tissue grinder, degrease with conventional organic solvents, such as 100mL of ethyl acetate, acetone or isopropyl ether, stir for 15 minutes to one hour, filter, and repeat washing 2 to 4 times to obtain The delipase powder is hydroxycyanolyase and stored in the refrigerator for later use.
[0047] After reacting sodium cyanide and glacial acetic acid, solvent extraction is used to obtain an organic solvent solution containing hydrocyanic acid, which is dried over anhydrous sodium sulfate and used; or directly adding hydrocyanic acid to an organic solvent containing 0-1% by weight of water.
Embodiment 2
[0049] 2) Synthesis of compound 2
[0050]
[0051] Add 400mg of compound 1, 0.15g of almond degreasing powder, 5ml of 1.5eq.HCN in isopropyl ether to a 25ml egg-shaped bottle, and react at 20°C for 24 hours. Filter, wash the enzyme source powder with ethyl acetate, and combine the organic phases. Organic phase with saturated FeCl 3 Aqueous wash to FeCl 3 The solution does not change color, Na 2 SO 4 dry. Colorless crystal product, yield 90%.
[0052] [α] D 20 +50.4(c0.83, CHCl 3 ), e.e.=96%;
[0053] 1 HNMR (CDCl 3 , 300MHz): δ, 2.50(s, 3H, CH 3 ), 3.00 (br, s, 1H, OH), 5.49 (s, 1H, CH), 7.29, 7.43 (AB, J 8.5Hz, 4H) ppm;
[0054] EI-MS: m / z (rel.intensty%): 181 (M + , 2, 5), 179 (M + , 79), 162 (M + -OH, 39), 153 (M + -CN, 55), 152 (M + -HCN, 100), 151 (M + -HCN-H, 88), 132(29), 123(16), 109(20), 105(17), 91(7), 77(17);
[0055] EA: Calculation: C: 60.31%, H: 5.06%, N: 7.82%
[0056] Found values: C: 60.40%, H: 5.06%, N: 7.73%
Embodiment 3
[0058] 3) Synthesis of compound 3
[0059]
[0060] Add compound 2 (0.9g), 2-Methoxypropene (20ml), CH to 250ml egg-shaped bottle 2 Cl 2 (150ml), POCl 3 (20 mg), stirred overnight at room temperature. Join Et 3 N 3mL, stirred for 30min, stopped. The organic phase was washed with saturated brine (50ml×3), anhydrous Na 2 SO 4 dry. Flash column chromatography (EA / PE=1 / 10) gave 1.09 g of a yellow solid with a yield of 96%. Recrystallization gave 0.92 g of colorless crystals with a yield of 77%.
[0061] 1 HNMR (CDCl 3 , 300MHz): δ, 1.4(s, 3H, CH 3 ), 1.58 (s, 3H, CH 3 ), 2.50(s, 3H, SCH 3 ), 3.2(s, 3H, OCH 3 ), 5.42 (s, 1H, CH), 7.25-7.41 (m, 4H, Hz, Ar-H) ppm, EI-MS: m / z (rel.intensity%): 251 (M + , 1.63), 219 (M + -H-OCH, 2.01), 179 (100.00), 162 (76.27), 151 (21.93), 132 (82.14), 109 (22.98), 105 (28.48), 86 (49.45), 45 (27.76);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com