Organic rare-earth ligand capable of regulating carrier transport power and its preparing method and use
A rare earth complex and transport capability technology, applied in chemical instruments and methods, lighting devices, light sources, etc., can solve the problems of low efficiency, no contribution to hole injection and transport, lack of practical prospects, etc., and achieve excellent application prospects, The effect of improving photoluminescence stability and improving electroluminescence efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
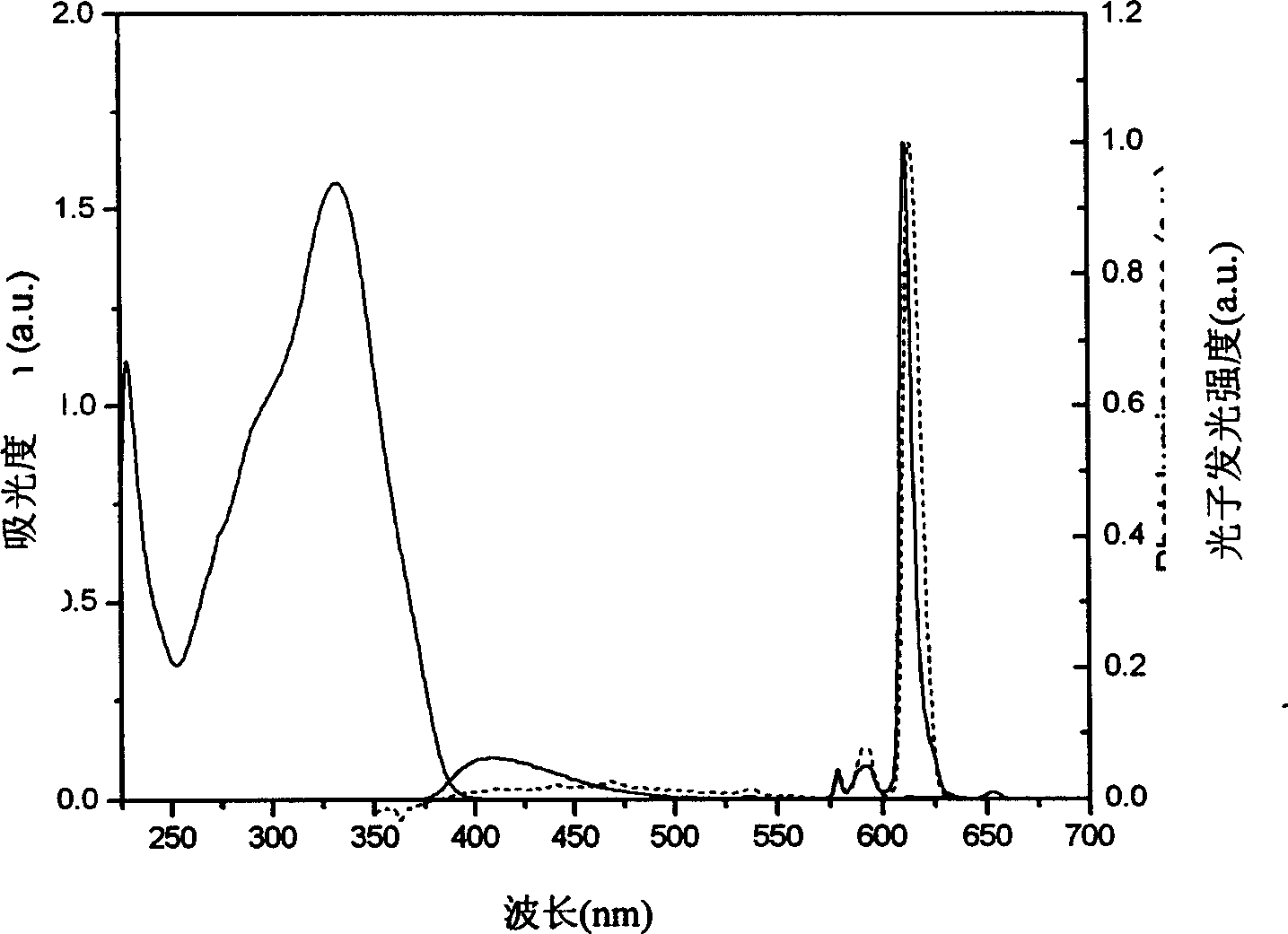
[0100] Embodiment 1, Eu(TTA) 3 (TAPO) 2 Synthesis
[0101] Add 333.3mg (1.5mmol) thienyl trifluoromethyl acetylacetone (TTA) dissolved in 15ml ethanol to the two-necked bottle, add dropwise 0.75ml 2M sodium hydroxide aqueous solution, heat to 60°C, reflux for 1h; weigh 183.2mg ( 0.5mmol) europium trichloride hexahydrate (EuCl 3 ·6H 2 O) Dissolved in 0.5ml of water, added dropwise to the system, and refluxed at 70°C for 1 hour; dissolved 455mg (1.0mmol) p-diphenylphosphine triphenylamine oxide (TAPO) in 15ml of ethanol, added dropwise to the system, and white Precipitation, reflux reaction overnight. Add dichloromethane for extraction, and recrystallize light yellow and slightly pink solid from a mixed solution of water and ethanol.
[0102] Anal.Calcd.for C 84 h 60 EuF 9 N 2 o 8 P 2 S 3 : C, 59.12; H, 3.54; Eu, 8.91; N, 1.64; O, 7.50; S, 5.64; Found: C, 59.01; .
[0103] ESI-MS (m / z): 854 (M 2+ ,41%); 891 (M 2+ +CH 3 CN, 78%); 446 (TAPO + , 100%).
Embodiment 2
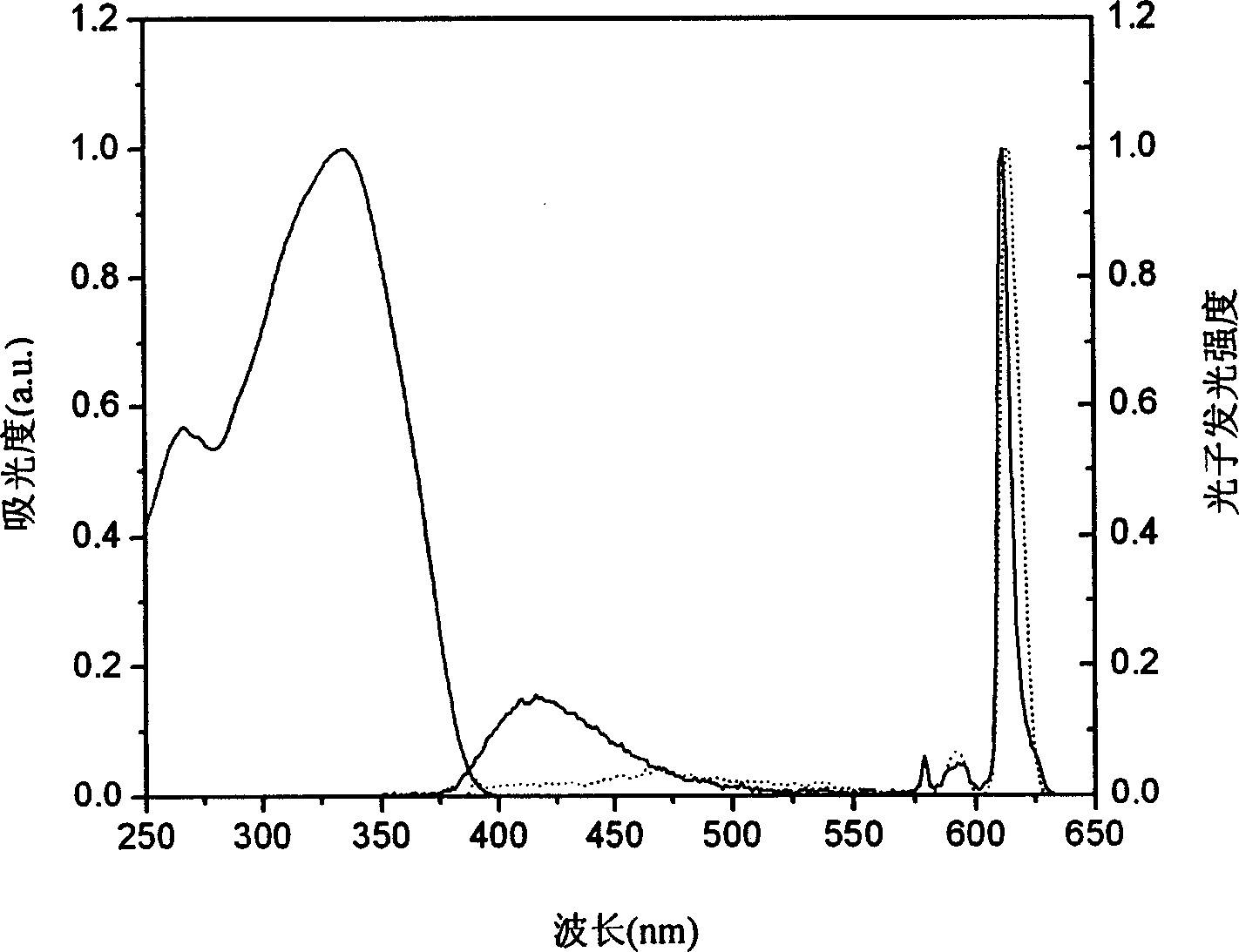
[0104] Example 2, Eu(TTA) 3 (NaDAPO) 2 Synthesis
[0105] Add 333.3mg (1.5mmol) thienyl trifluoromethyl acetylacetone (TTA) dissolved in 15ml ethanol to the two-necked bottle, add dropwise 0.75ml 2M sodium hydroxide aqueous solution, heat to 60°C, reflux for 1h; weigh 183.2mg ( 0.5mmol) europium trichloride hexahydrate (EuCl 3 ·6H 2 O) dissolved in 0.5ml of water, added dropwise to the system, and refluxed at 70°C for 1h; 495mg (1.0mmol) of N-naphthyl-p-diphenylphosphine diphenylamine oxide (NaDAPO) was dissolved in 15ml of ethanol, and added dropwise to In the system, a white precipitate appeared, and the reaction was refluxed overnight. Add dichloromethane for extraction, and recrystallize light yellow and slightly pink solid from a mixed solution of water and ethanol.
Embodiment 3
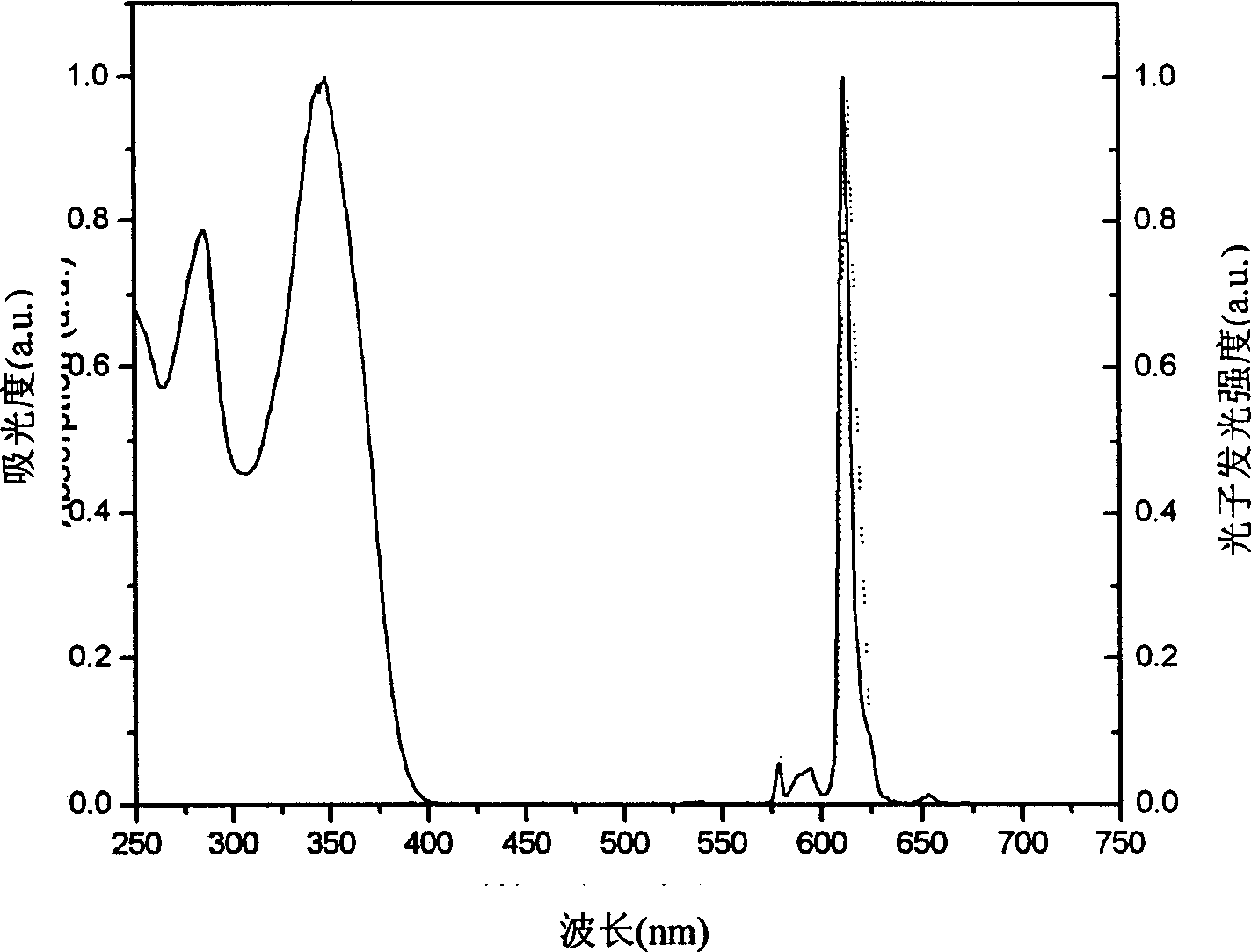
[0106] Embodiment 3, Eu(TTA) 3 (CPO) 2 Synthesis
[0107] Add 333.3mg (1.5mmol) thienyl trifluoromethyl acetylacetone (TTA) dissolved in 15ml ethanol to the two-necked bottle, add dropwise 0.75ml 2M sodium hydroxide aqueous solution, heat to 60°C, reflux for 1h; weigh 183.2mg ( 0.5mmol) europium trichloride hexahydrate (EuCl 3 ·6H 2 O) Dissolved in 0.5ml of water, added dropwise to the system, and refluxed at 70°C for 1h; dissolved 367mg (1.0mmol) of N-diphenylphosphine carbazole (CPO) in 15ml of ethanol, added dropwise to the system, and White precipitate was refluxed overnight. Add dichloromethane for extraction, and recrystallize light yellow and slightly pink solid from a mixed solution of water and ethanol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com