Polymer material containing anthracene and diketopyrrolopyrrole units and its preparation method and application
A technology of diketopyrrolopyrrole and polymer materials, applied in the field of organic semiconductor materials, can solve the problems of affecting the efficiency of exciton diffusion, reducing the photoelectric conversion efficiency of organic solar cells, poor solubility, etc., and achieving high photoelectric conversion efficiency and convenient The effect of promoting the application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
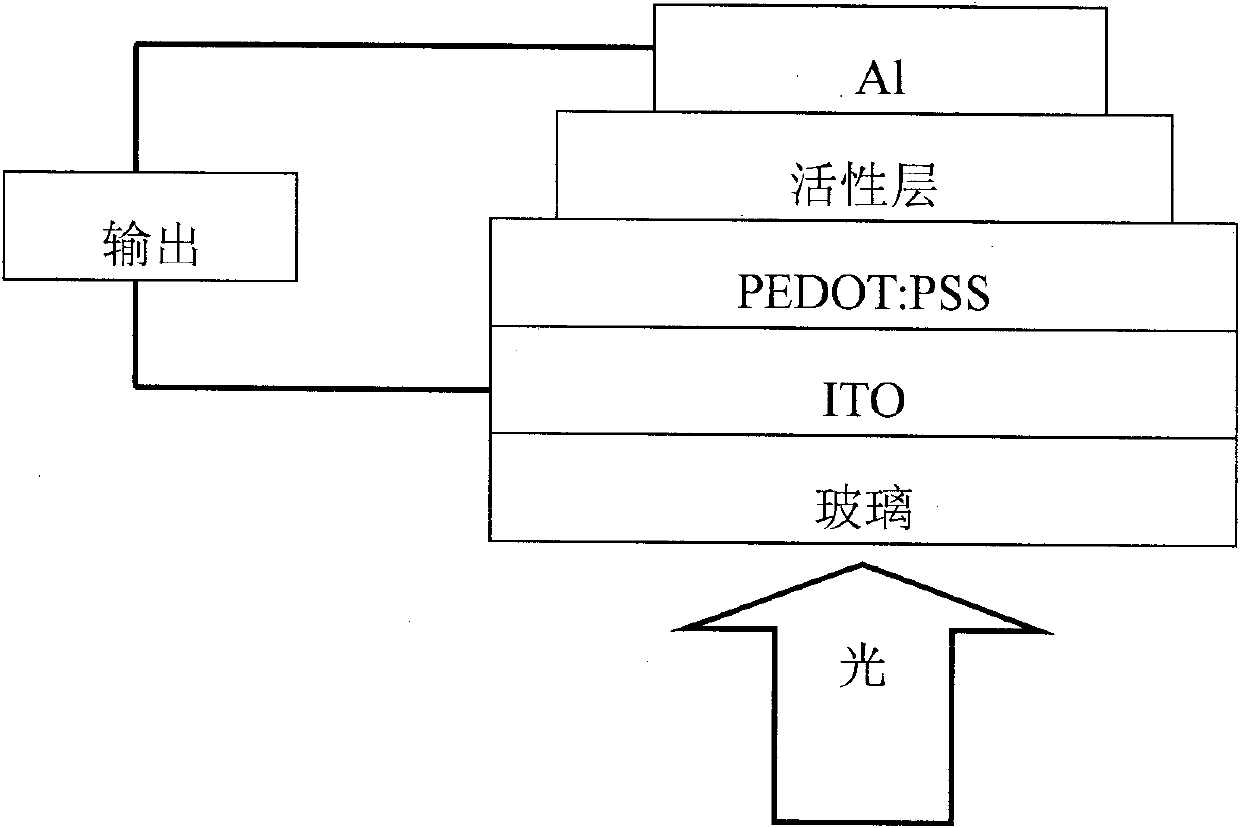
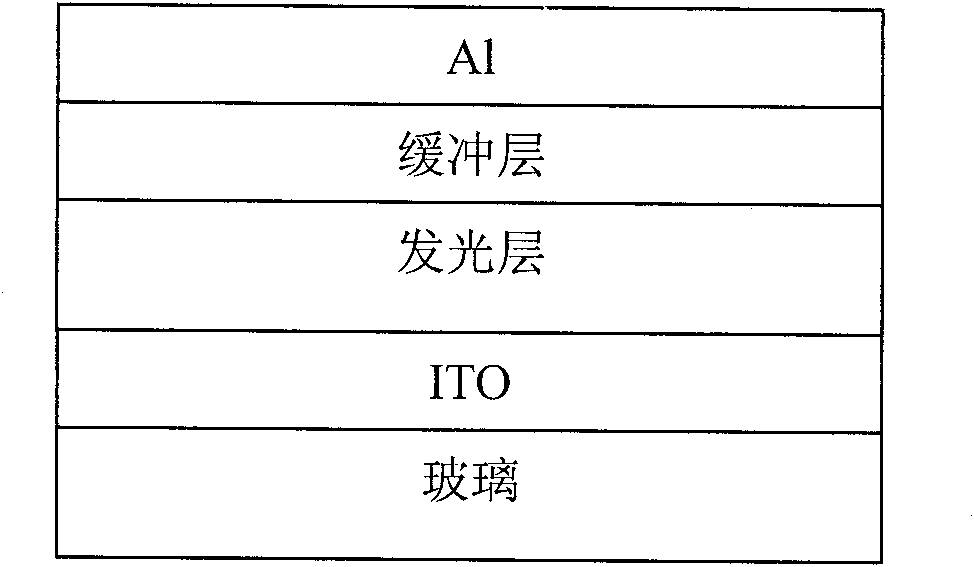
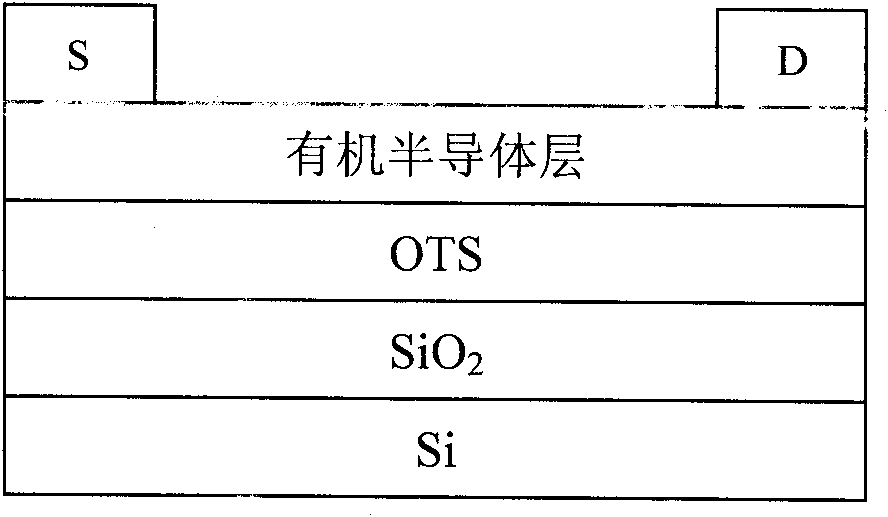
Image
Examples
Embodiment 1
[0042] Example 1 This example discloses polymer P1 with the following structure:
[0043]
[0044] The preparation steps of P1 are as follows:
[0045] Step 1, the preparation of 9,10-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) base anthracene:
[0046]
[0047] Set up the tetrahydrofuran (THF) distillation device, add the tetrahydrofuran solvent that has been dried with KOH for several days into a 1000ml single-necked bottle, then cut into the sodium block and add benzophenone, keep stirring and reflux until the system is dark purple, evaporate Refined THF is ready for use.
[0048] Set up an anhydrous and oxygen-free reaction device, under the protection of constant stirring and N2, add 9.0mmol of light yellow crystal 9,10-dibromoanthracene into the three-necked flask, inject 150ml of the above-mentioned refined tetrahydrofuran solvent with a syringe, and in- Slowly inject 27.0mmol n-BuLi with a syringe at 75°C, the system gradually changes from light yellow to oran...
Embodiment 2
[0070] Example 2 This example discloses polymer P2 with the following structure:
[0071]
[0072] The preparation steps of P2 are as follows:
[0073] Step 1, the preparation of 9,10-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) base anthracene:
[0074] For its preparation, see Step 1 in Example 1.
[0075] Step 2, the preparation of 3,6-dithienyl-diketopyrrolopyrrole:
[0076] For its preparation, refer to Step 2 in Example 1.
[0077] Step 3, the preparation of 2,5-dioctyl-3,6-dithienyl-pyrrolopyrrole diketone:
[0078] For its preparation, see Step 3 in Example 1.
[0079] Step 4. Preparation of 2,5-dioctyl-3,6-bis(5-bromothiophene)yl-diketopyrrolopyrrole:
[0080] For its preparation, see Step 4 in Example 1 for details.
[0081] Step 5. Preparation of 2,5-dioctyl-3,6-bis(4-dodecyldithiophene)yl-diketopyrrolopyrrole:
[0082]
[0083] Dissolve 1 mmol of 2,5-dioctyl-3,6-bis(5-bromothiophene)-pyrrolopyrrole diketone and 2.3 mmol of 4-dodecyl-2-tributyltin-based...
Embodiment 3
[0092] Example 3 This example discloses polymer P3 with the following structure:
[0093]
[0094] The preparation steps of P3 are as follows:
[0095] Step 1. 9,10-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-2,6-bis(2-octyldecyl)anthracene Preparation of:
[0096]
[0097] Set up an anhydrous and anaerobic reaction device, under the protection of constant stirring and N2, add 9,10-dibromo-2,6-bis(2-octyldecyl)anthracene 5mmol in the three-necked flask (the compound method Refer to Klaus Mullen et al's Macromol.Chem.Phys.2006, 207, 1107-1115), inject 150ml of refined tetrahydrofuran solvent with a syringe, slowly inject 15mmol n-BuLi with a syringe at -80°C, and stir the reaction 2 Hours; after 2 hours of reaction, inject 15mmol 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane with a syringe at -80°C, and raise the temperature React overnight at room temperature.
[0098] After the reaction, add saturated aqueous sodium chloride solution, extract with chloroform,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com