Organic semiconductor laser material as well as preparation method and application thereof
A technology of organic semiconductors and laser materials, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of high optical gain medium threshold, complex material preparation, high water and oxygen sensitivity, and achieve high luminous efficiency and migration. efficiency, simple preparation method, and high luminous intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
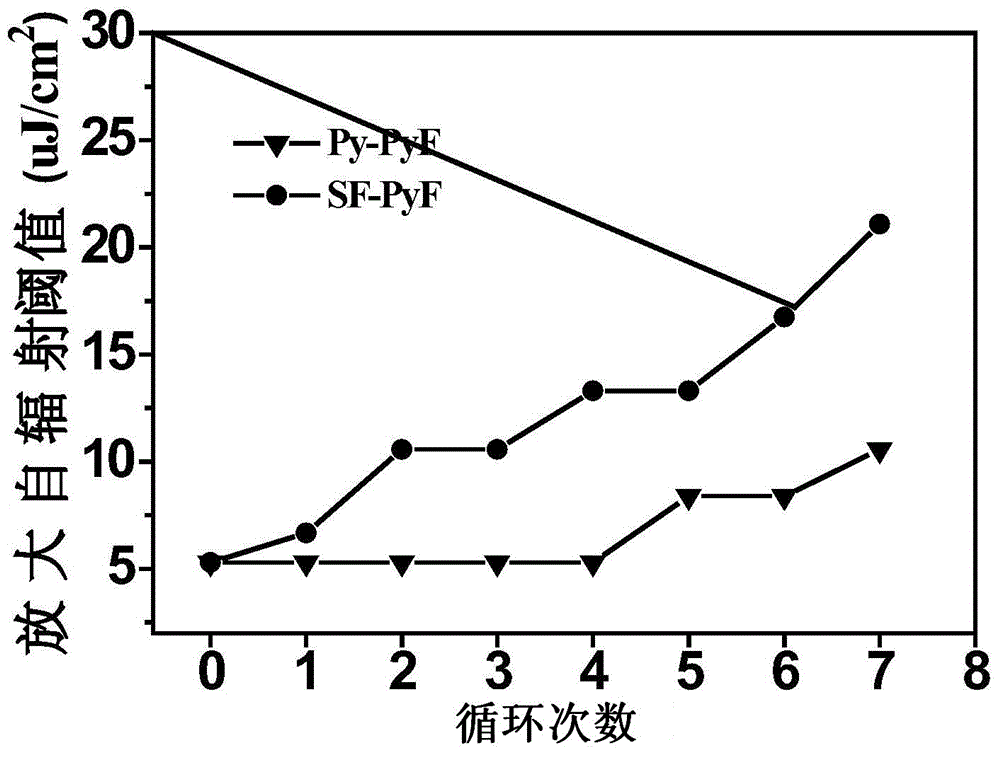
[0029] The preparation method of the present invention comprises: 1-bromopyrene and 2,7-dibromofluorene synthesize the monobromo-substituted derivatives of pyrenylfluorene through Suzuki reaction, then carry out boron esterification reaction, and finally combine it with different four-substituted nuclei Compound I was prepared by Suzuki coupling reaction. The four-substituted butterfly-shaped organic semiconductor laser material based on pyrenylfluorene as the arm has low threshold, low water and oxygen sensitivity and high thermal stability. It has a structure such as formula I:
[0030]
[0031] Wherein, the nucleus A in formula I is one of the following II structures:
[0032]
[0033] Wherein, R is a C1-C20 linear or branched alkyl or alkoxy group; * is a connection position; O is an oxygen atom; N is a nitrogen atom; H is a hydrogen atom.
[0034] The preparation method of the butterfly-shaped organic semiconductor laser material comprises the following steps: ...
Embodiment 1
[0041]
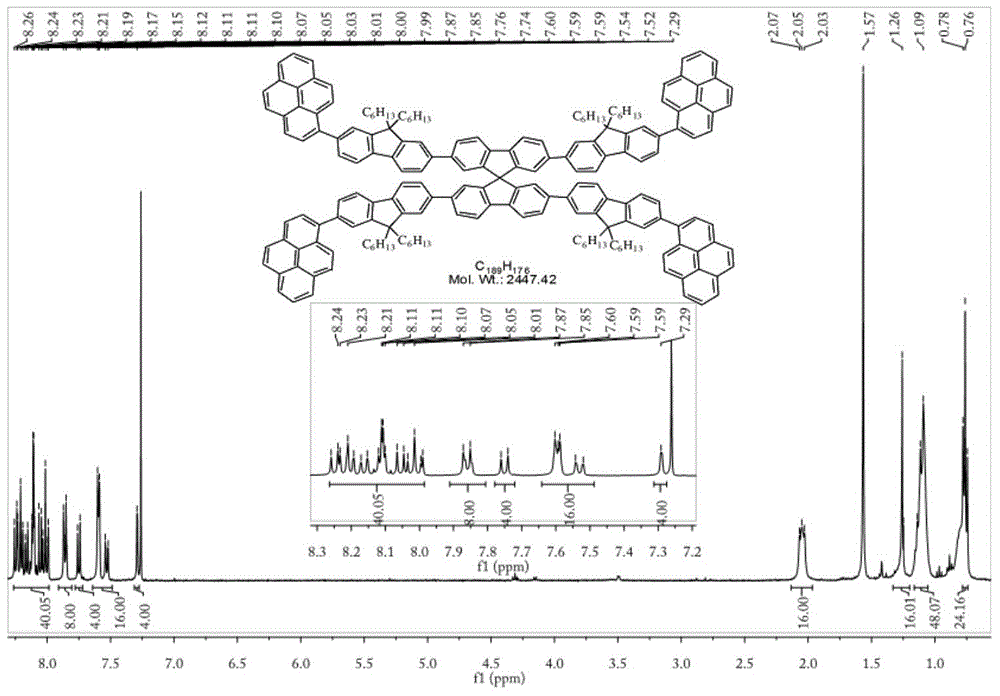
[0042] Reaction condition one: in N 2 Under protection, pyrenyl-1-boronic acid pinacol ester (328.2mg, 1mmol), 9,9-dihexyl-2,7-dibromofluorene (984.6mg, 2mmol) was added to a 150mL reaction flask, and N 2 After three times, Pd(PPh 3 ) 4 Catalyst (57.8mg, 0.05mmol) and phase transfer catalyst TBAB (32.2mg, 0.1mmol) were dissolved in 21mL of toluene and 7mL of K 2 CO 3 In the aqueous solution, the temperature was controlled at 80° C. for 12 hours; after the reaction, the product was purified by chromatographic column to obtain the product of structural formula 1 (343.7 mg), and the yield was 56.1%.
[0043] Reaction condition two: in N 2 Under protection, add pyrenyl-1-boronic acid pinacol ester (328.2mg, 1mmol), 9,9-dihexyl-2,7-dibromofluorene (1.231g, 2.5mmol) into a 150mL reaction flask, and replace N 2 After three times, Pd(PPh 3 ) 4 Catalyst (115.6mg, 0.10mmol) and phase transfer catalyst TBAB (48.3mg, 0.15mmol) were dissolved in 42mL of toluene and 14mL...
Embodiment 2
[0047]
[0048] Reaction condition 1: Under the condition of nitrogen protection, 1-(7-bromo-9,9-dihexyl-9H-fluoren-2-yl)pyrene (613.7mg, 1mmol) and diboronic acid pinacol ester (457.1g, 1.8mmol), Pd(dppf 2 )Cl 2 Catalyst (40.8mg, 0.05mmol) and KOAc (196.0mg, 2mmol) were dissolved in 20mL of anhydrous dioxane solvent, under temperature control at 90°C, and reacted in the dark for 24h; after the reaction was completed, 1-( 7-boronic acid pinacol ester-9,9-dihexyl-9H-fluoren-2-yl)pyrene (366.0 mg), yield 55.4%.
[0049] Reaction condition 2: Under the condition of nitrogen protection, 1-(7-bromo-9,9-dihexyl-9H-fluoren-2-yl)pyrene (613.7mg, 1mmol) and diboronic acid pinacol ester (506.1g, 2.0mmol), Pd(dppf 2 ) Cl 2 Catalyst (81.6mg, 0.10mmol) and KOAc (294.0mg, 3mmol) were dissolved in 35mL of anhydrous dioxane solvent, and the temperature was controlled at 100°C, and reacted in the dark for 30h; after the reaction was completed, 1-( 7-boronic acid pinacol ester-9,9-dihex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com