Beta-carotene clathrate and its preparation method
A carotene and clathrate technology, which is applied in the field of clathrates composed of β-carotene and β-cyclodextrin derivatives and its preparation, can solve the problems of poor solubility and achieve improved solubility and high inclusion rate , the effect of facilitating industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: The present invention prepares β-carotene and HP-β-CD solid clathrate
[0020] Accurately weigh 0.02g of β-carotene and HP-β-CD1g and place them together in a mortar, add 1ml of distilled water dropwise, and grind under the protection of nitrogen. After grinding for 3 hours, a red paste was obtained. Dissolve the red paste with 100ml of ethanol, stir at low speed for 6 hours at room temperature, distill off the organic solvent under reduced pressure, add distilled water to redissolve, and let stand at low temperature for 5 hours. After concentrating under reduced pressure, vacuum-dry at 50°C for 5 hours to obtain a deep red solid clathrate. The content (w / w) of β-carotene in the clathrate was determined by ultraviolet-visible spectrophotometry=1.7%, the yield=82%, and the inclusion rate=95%.
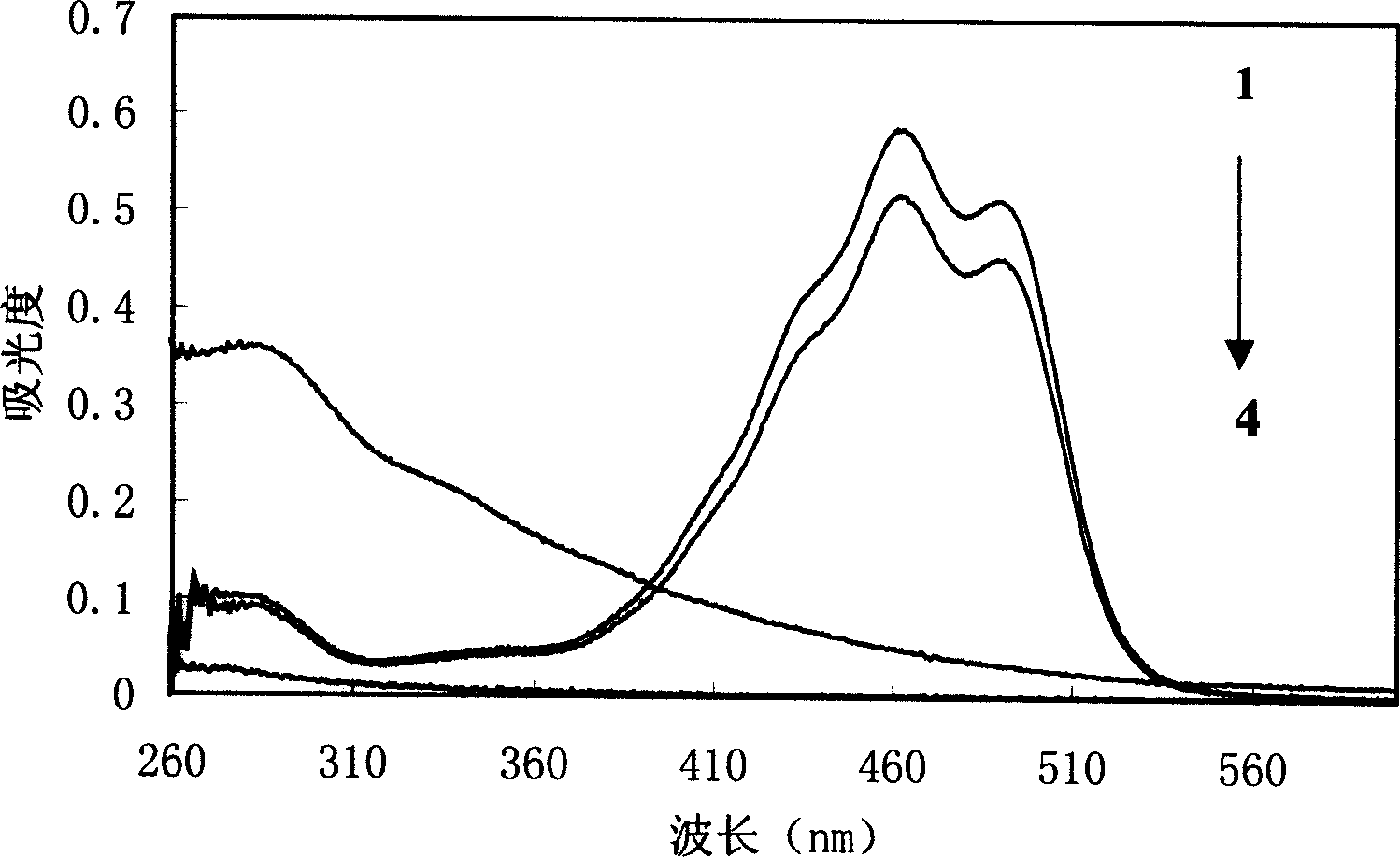
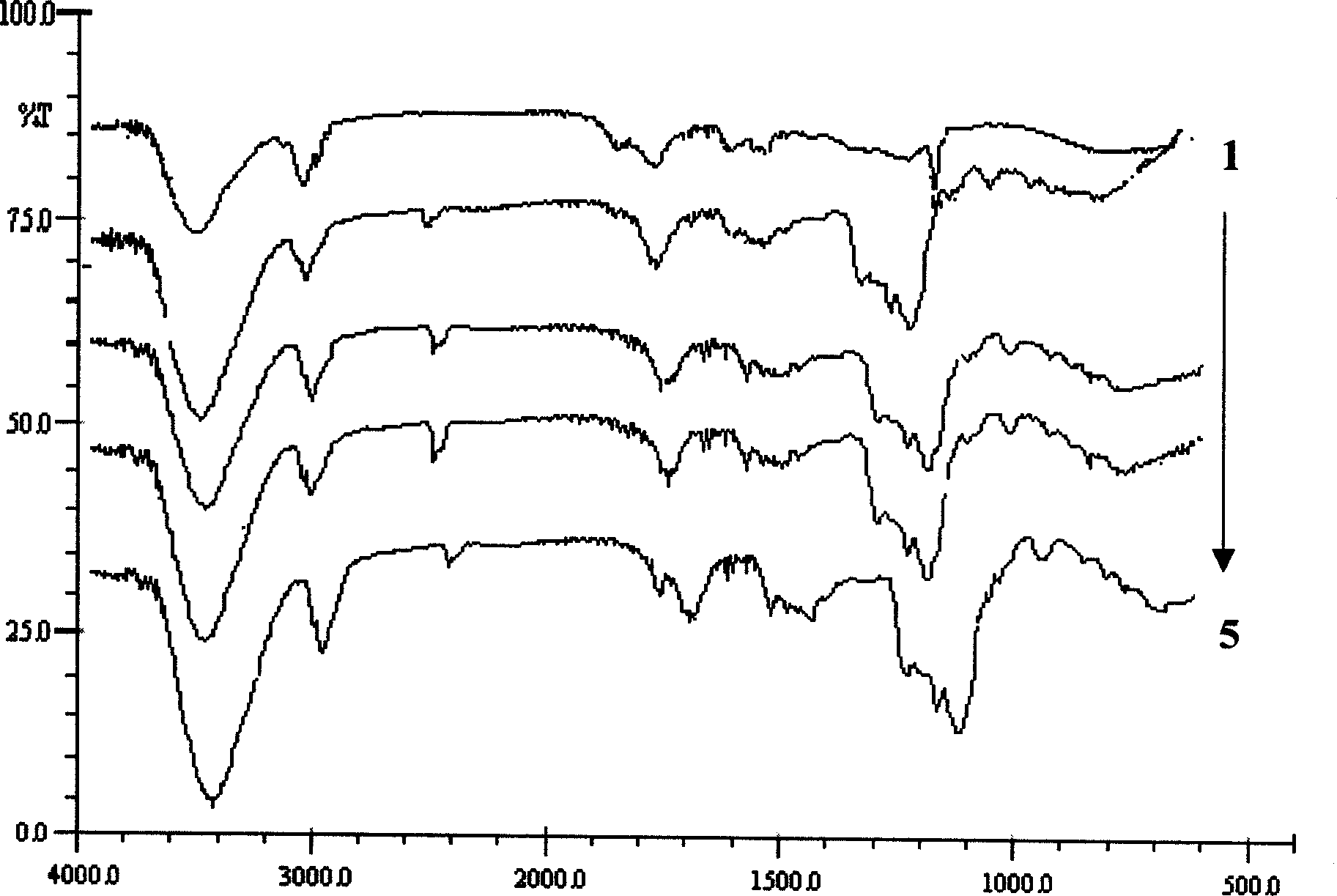
[0021] Using ultraviolet-visible spectrophotometry, it is determined that the inclusion substance in the inclusion complex is β-carotene. Extract the clathrate with D...
Embodiment 2
[0022] Example 2: The present invention prepares β-carotene and HP-β-CD solid clathrate
[0023] Accurately weigh 0.05 g of β-carotene and 0.45 g of HP-β-CD and place them together in a mortar, add 1 ml of distilled water dropwise, and grind under the protection of nitrogen. After grinding for 2 hours, a red paste was obtained. Dissolve the red paste with 50ml of ethanol, stir at low speed for 6 hours at room temperature, distill off the organic solvent under reduced pressure, add distilled water to redissolve, and let stand at low temperature for 5 hours. After concentrating under reduced pressure, vacuum-dry at 50°C for 5 hours to obtain a deep red solid clathrate. The content (w / w) of β-carotene in the inclusion compound was determined by ultraviolet-visible spectrophotometry=6.21%, the yield=82%, and the inclusion rate=97%.
[0024] Infrared spectroscopy was used to determine that the inclusion substance in the inclusion compound was β-carotene. The infrared spectrum of ...
Embodiment 3
[0025] Example 3: The present invention prepares β-carotene and SBE-β-CD solid clathrate
[0026] Accurately weigh 0.02g of β-carotene and 0.4g of SBE-β-CD and place them together in a mortar, add 1ml of distilled water dropwise, and grind under the protection of nitrogen. After grinding for 3 hours, a red paste was obtained. Dissolve the red paste with an appropriate amount of water, stir at low speed for 6 hours at room temperature, and stand at low temperature for 5 hours. After concentrating under reduced pressure, it was vacuum-dried at 35°C for 5 hours to obtain a dark red solid clathrate. The content (w / w) of β-carotene in the inclusion compound was determined by ultraviolet-visible spectrophotometry=6.4%, the yield=73%, and the inclusion rate=98.56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com