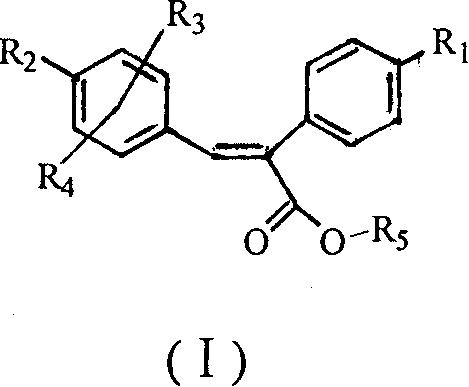
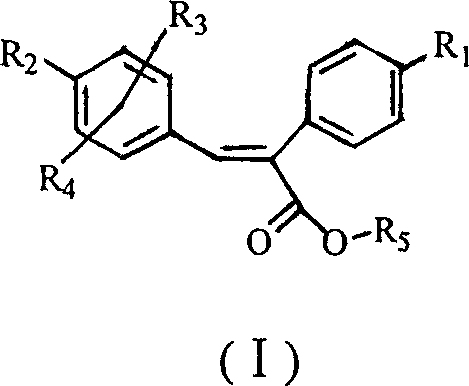
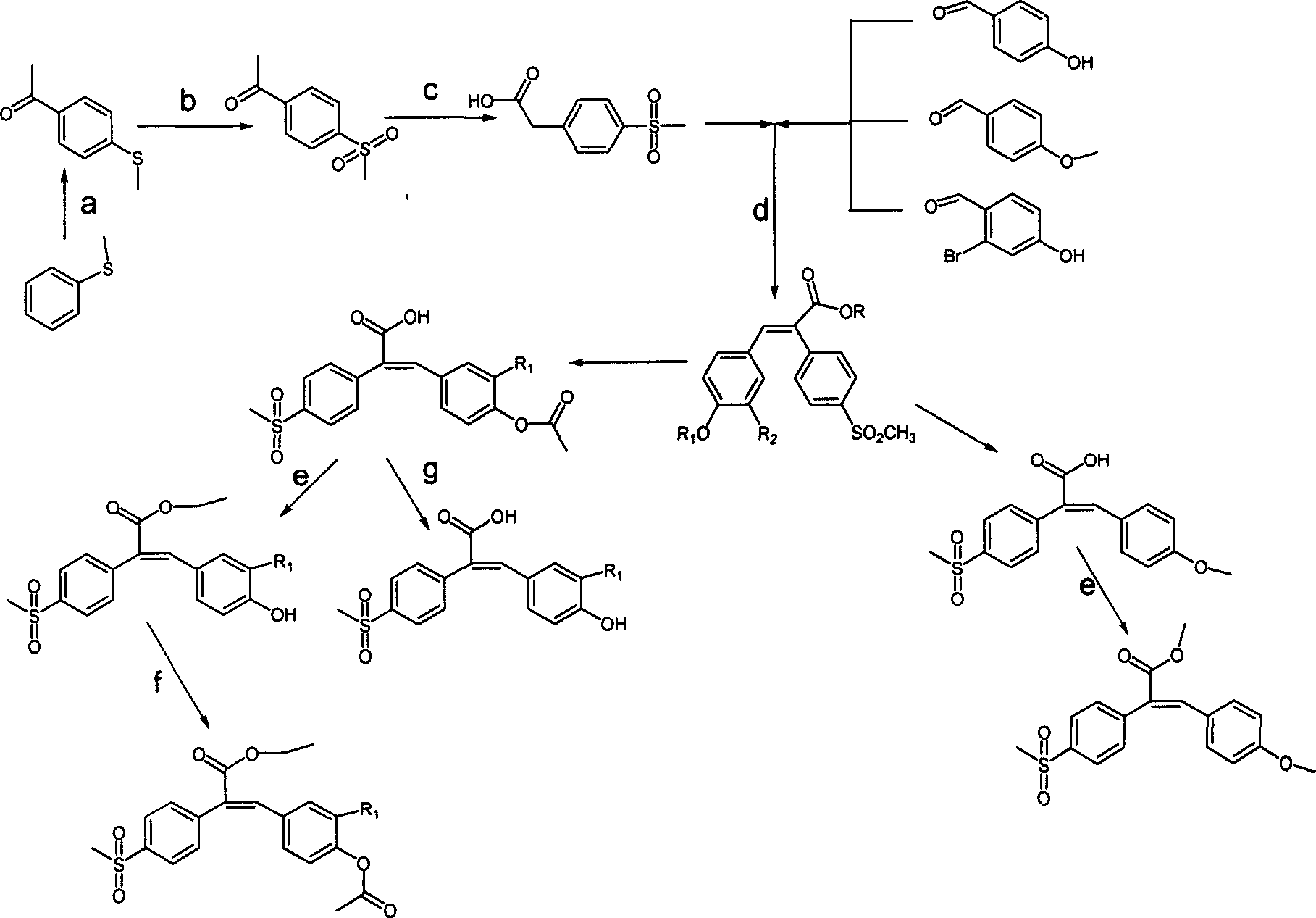
Sulfonyl diphenylethyllene endocompound and its preparation method and pharmaceutical uses
A compound, methanesulfonyl technology, applied in the field of ethylene bridge compounds, can solve the problems that have not yet been seen, and achieve the effect of good anti-inflammatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: the preparation of 4-methylsulfide acetophenone
[0069]
[0070] In a 1L four-necked bottle, add AlCl 3 130.0g (0.97mol) and 650mL of chloroform, cooled in an ice-water bath to about 5°C, stirred and added dropwise 76g (0.97mol) of acetyl chloride, after the dropwise addition, kept the temperature at 5°C, and added dropwise under stirring within 1hr Thioanisole 100g (0.81mol), dropwise completed, removed from the ice-water bath, stirred at room temperature for 2hr, poured into a mixture of 1000mL water, 250mL concentrated HCl and 500g ice, stirred, separated the chloroform layer, and extracted with 100mL chloroform The aqueous layer was combined with the chloroform layer, dried with anhydrous sodium sulfate, evaporated in an oil bath at 100°C to remove chloroform, and then evaporated to dryness under reduced pressure to obtain a light yellow liquid, which was removed from the oil bath and cooled to become a solid with a weight of 127.0 g, yield 95.0%...
Embodiment 2
[0072] Embodiment 2: the preparation of 4-methylsulfoneacetophenone
[0073]
[0074]In a 500mL four-neck flask, add 83.1g (0.5mol) of 4-methylsulfide acetophenone, 180mL of acetic acid and 1.0mL of 85% phosphoric acid, put it in an oil bath at 110°C, heat and stir, and drop Add 30%H 2 o 2 A mixture of 130mL (1.1mol) and 0.7mL of 85% phosphoric acid, after the dropwise addition, was stirred in an oil bath for 1hr, then poured into a mixture of 600mL of water and 300g of ice, stirred to precipitate a white solid, cooled and filtered to obtain a white solid , washed twice with water, then twice with ethanol, and dried to obtain 95.0 g of white solid with a yield of 96.0%. Recrystallized with ethanol to obtain white prismatic crystals, m.p.126.2-126.5°C. (Literature melting point 124-126°C).
[0075] IR(KBr)(v / cm -1 ): 3356.33, 3095.29, 3072.47, 3017.05, 3004.23, 2922.78, 1687.69 (υ c=o ), 574.31, 1395.36, 1356.83, 1310.57, 1296.21, 1258.55, 1172.40, 1148.46.1089.72
Embodiment 3
[0076] Embodiment 3: the preparation of 4-methylsulfone phenylacetic acid
[0077]
[0078] In a 1L four-neck flask, add 80g (0.4mol) of 4-methylsulfoneacetophenone, 12.8g (0.4mol) of sublimed sulfur and 56.0mL of morphineline, then heat to 130°C and reflux for 10hr, cool to 50°C, and Add 80mL of ethyl acetate, stir evenly, then add 40mL of petroleum ether, stir for about 20 minutes, it becomes solid, then add 80g of NaOH in 800mL of water solution, place in an oil bath at 85°C and stir overnight, remove from the oil bath , cooled to room temperature, filtered, added 160mL of concentrated hydrochloric acid, adjusted the pH to about 4-5, precipitated a light yellow solid, and recrystallized the solid with ethyl acetate to obtain 43.0 g of colorless needle crystals, yield 50.2%, m.p.134-136 ℃. (Literature melting point 137°C).
[0079] IR(KBr)(v / cm -1 ): 3095.17, 3070.50, 3030.96, 3016.14, 2930.92, 2732.30, 1699.96 (υ c=o ), 1409.51, 1297.81, 1240.15, 1145.54, 1090.27, 96...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com