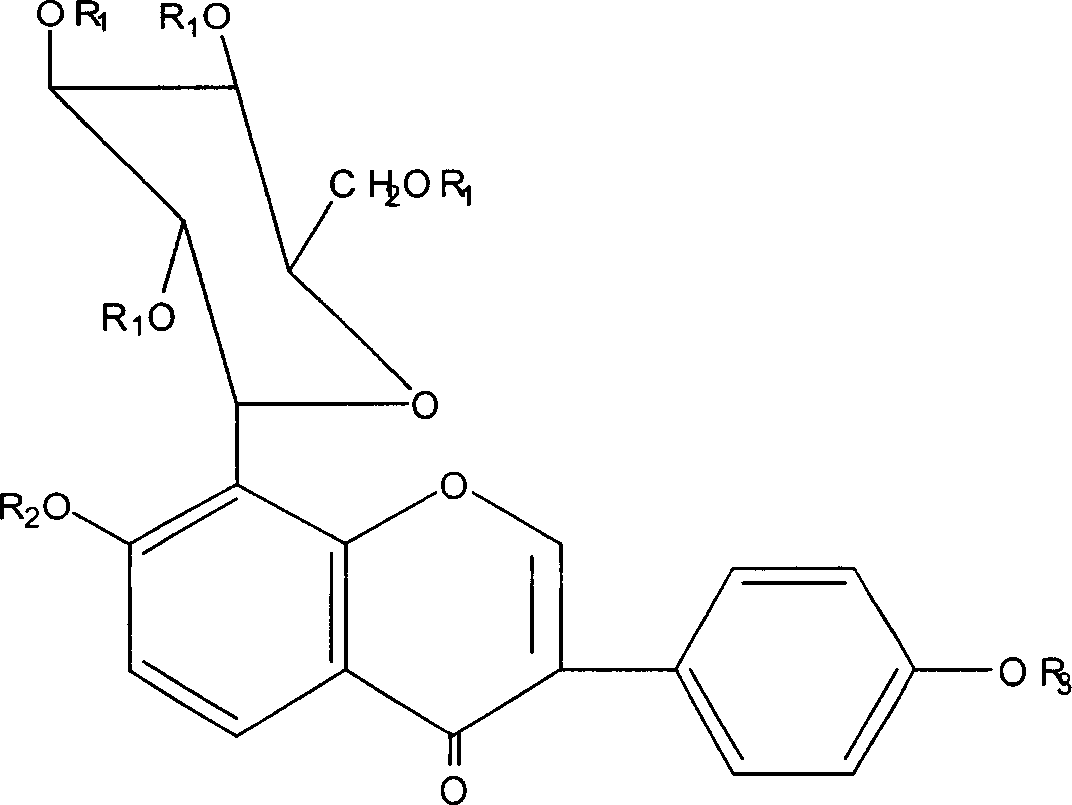
Puerarin derivatives and their medical uses
A technology of puerarin and tetraacetyl puerarin, applied in the field of puerarin derivatives, can solve the problems of low bioavailability, limited use, and limited clinical application range of puerarin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
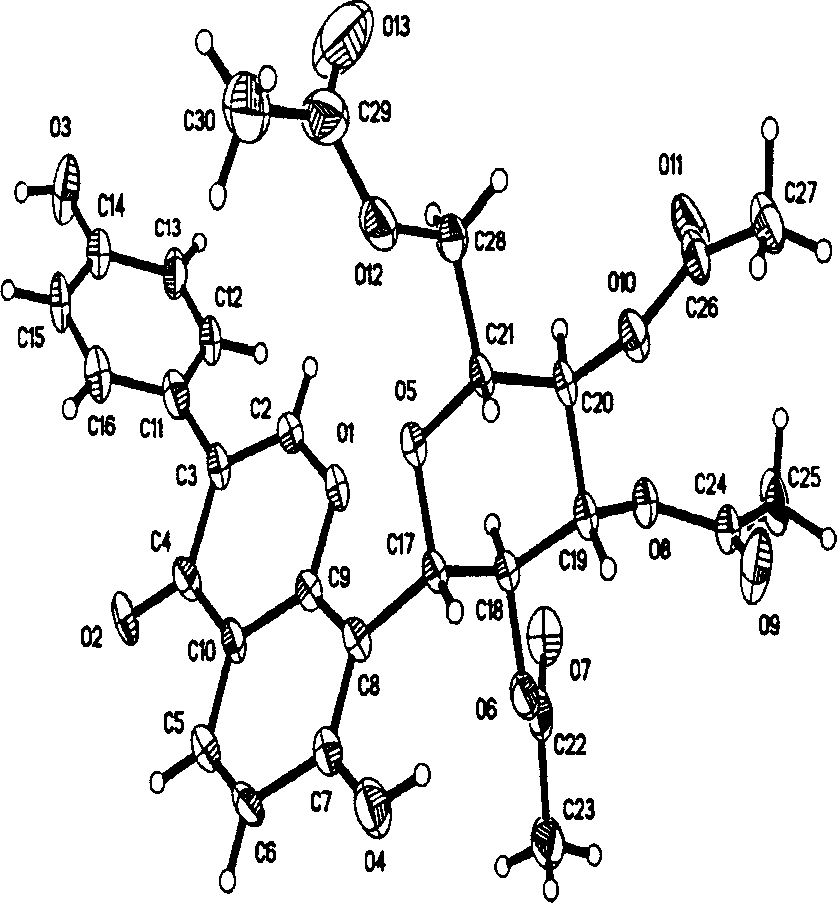
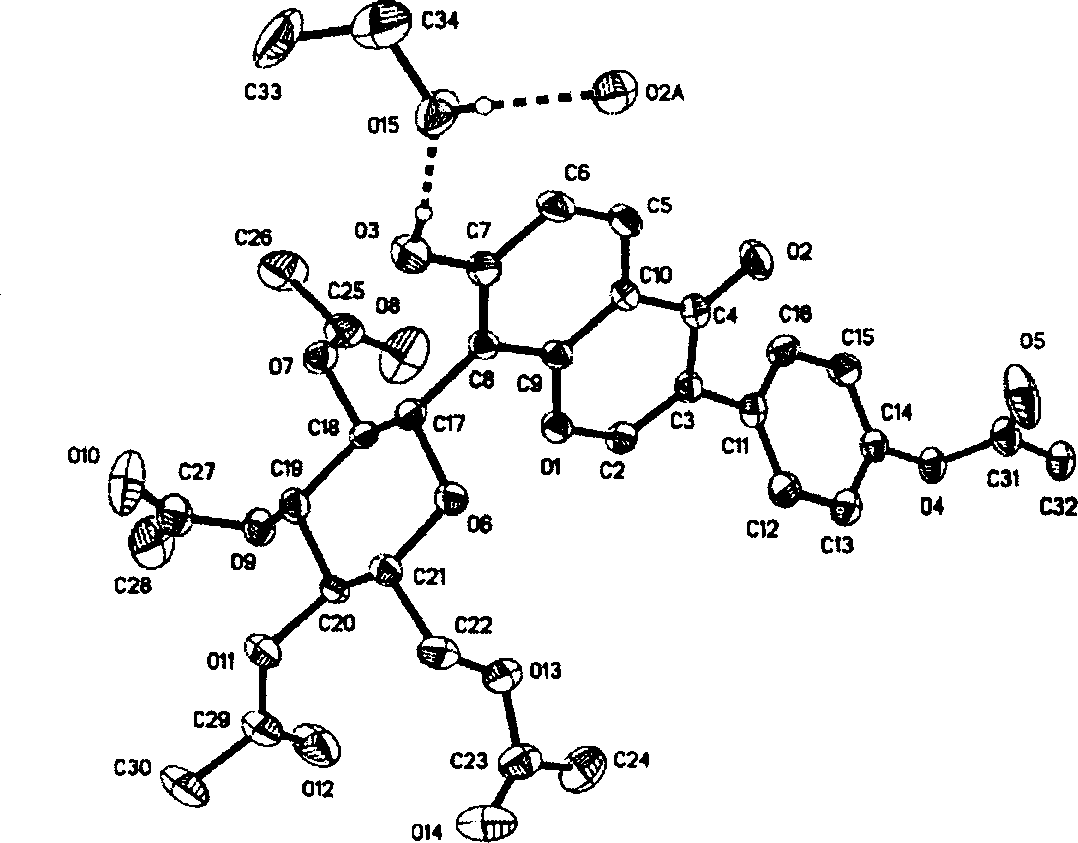
Image
Examples
Embodiment 1
[0019] Synthesis and structure identification of embodiment 1 compound I-III
[0020] 1.1 The synthesis of compound I-III comprises the following steps:
[0021] (1) 50g of puerarin (purity 99%, Shaanxi Huike Plant Technology Co., Ltd., China) was dissolved in 600ml of pyridine, and 100g of anhydrous acetic anhydride was added, stirred at room temperature for 30 minutes, and left at room temperature for 24 hours to obtain the mixture That is reactant A.
[0022] (2) Slowly pour reactant A into 10L of ice water, stir well and filter under reduced pressure to obtain approximately 80 g of reactant B.
[0023] (3) Dissolve reactant B in dichloromethane and add 5% sodium carbonate solution, fully stir at room temperature for 1 hour, separate the organic phase, and evaporate to obtain about 70 g of anhydrous reactant C.
[0024] (4) C is poured into a chromatographic column equipped with 5000 g of silica gel H (10-40 μ, Qingdao Ocean Chemical Factory). The eluent is a mixture o...
example 2
[0060] Example 2: Study on the bioavailability of orally administered puerarin derivatives:
[0061] 2.1 Materials
[0062] 2.1.1 Agents and reagents
[0063] Puerarin was purchased from Beijing United Pharmaceutical Factory (People's Republic of China, PRC, lot number: 030404). The purity was 99% by high performance liquid chromatography (HPLC). Puerarin derivatives 4ac, 5ac, and 6ac can be synthesized by the above method, provided by Hong Kong Polytechnic University, and their analyzed purity is over 98%. Both acetonitrile and methanol were of HPLC grade and double distilled water was used.
[0064] 2.1.2 Instrument and chromatographic conditions
[0065] Agilent1100HPLC, DAD diode, HP1100 chromatography workstation, AgilentXDB-C 18 Column (250mm×4.6mmD, 5μm), the pre-chromatographic column is AgilentXDB-C 18 Column (12.5 mm x 4.6 mm D, 5 μm). Gradient elution is carried out according to the following table 1:
[0066] Table 1 Chromatographic conditions
[0067] ...
Embodiment 3
[0109] Embodiment 3: The effect of puerarin and its derivatives in the treatment of the disease caused by the injection of the posterior pituitary extract Efficacy of acute myocardial ischemia in rats
[0110] 3.1 Materials
[0111] 3.1.1 Test compounds
[0112] Puerarin was purchased from Beijing United Pharmaceutical Factory (China). Derivatives 4ac, 5ac and 6ac of puerarin were provided by Hong Kong Polytechnic University. Puerarin was dissolved in 1:1 PEG400 and sterilized distilled water to prepare 0.8g / kg body weight and 10ml / kg body weight solution for intragastric feeding .
[0113] The puerarin derivative 4ac was dissolved in 1:1 PEG400 and sterile distilled water to prepare a 1.12 g / kg, 10 ml / kg solution for intra-gastro feeding. Dissolve 5ac in 1:1 PEG400 and sterile distilled water to prepare 1.20g / kg, 10ml / kg solution for intragastric feeding. Dissolve 6ac in 1:1 PEG400 and sterile distilled water to prepare 1.28g / kg, 10ml / kg solution for intragastric feed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com