Puerarin oral preparation and its preparation method
An oral preparation, puerarin technology, applied in the field of medicine, can solve the problems of a long treatment course for cardiovascular and cerebrovascular diseases, increase the pain of patients, there is no public report on oral preparations of puerarin, etc., and achieves improved oral bioavailability and increased permeability. Sexual and qualitatively stable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
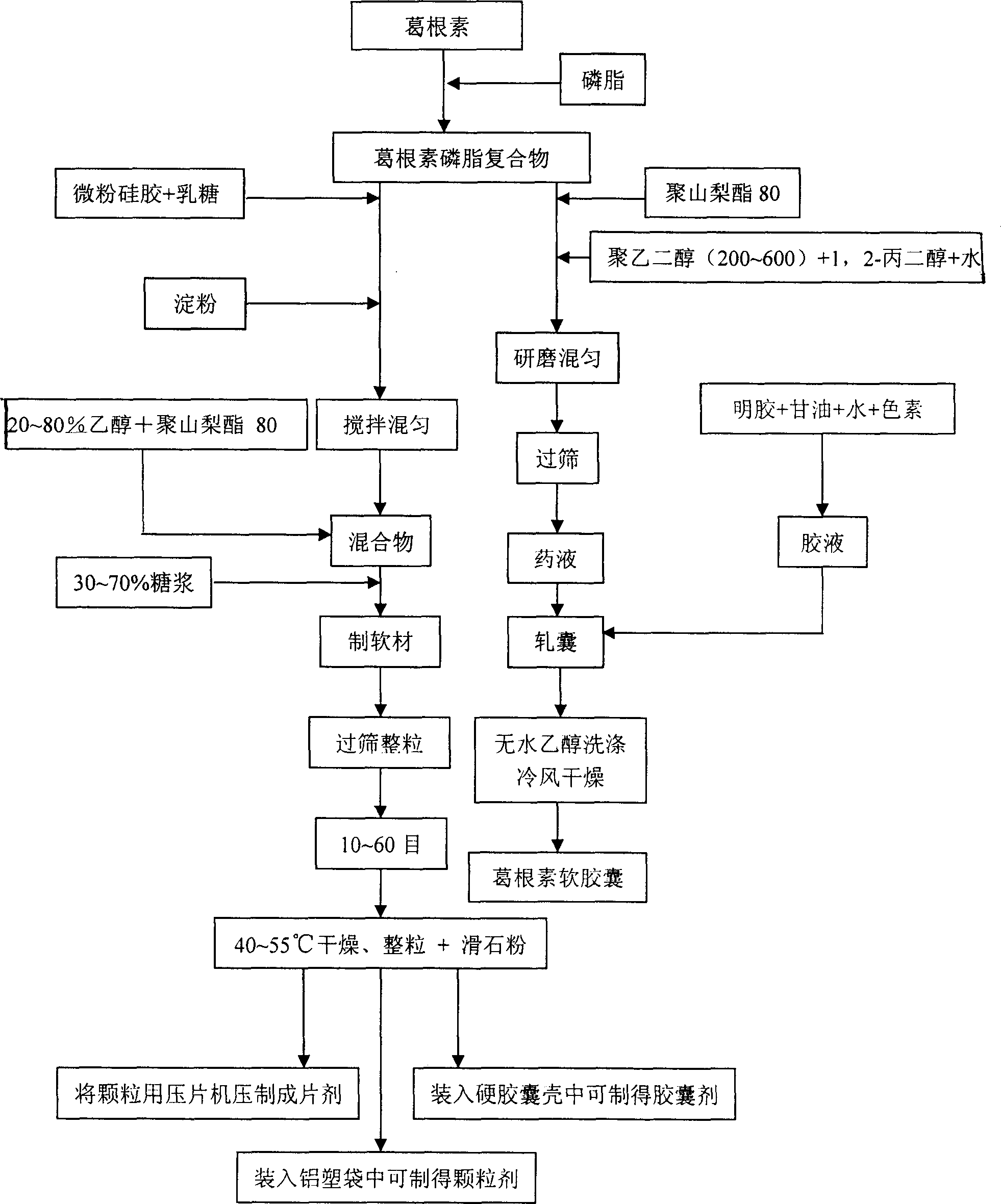
[0024] Embodiment 1: puerarin granules
[0025] Put puerarin and soybean lecithin in a ratio of 2:1 by weight, and dissolve them in 10 times acetone under the condition of reflux and stirring at 50°C. After reacting for 120 minutes, evaporate the solvent to dryness, and dry in vacuum at 50°C for 10 hours to obtain the puerarin-phospholipid complex. Grind through a 120-mesh sieve; mix 150g of puerarin-phospholipid complex with 600g of micropowdered silica gel, 1500g of lactose, and 600g of starch, and then mix 1,000g of 50% ethanol and 180g of polysorbate 80 (molecular weight: 1309.7) and add to the mixture , mix evenly, then use 200g of 70% syrup to make the soft material of binder system, pass through 20 mesh sieves to make granules, dry at 55°C for 6 hours, cross 20 mesh sieves for granulation, and measure the content of puerarin in the granules by HPLC. Add 3 g of talcum powder, pack and seal 0.1 g of puerarin per bag to obtain puerarin granules.
Embodiment 2
[0026] Embodiment 2: puerarin granules
[0027] Put puerarin and soybean lecithin in a ratio of 1:1 by weight, and dissolve them in 10 times absolute ethanol under reflux and stirring at 50°C. After reacting for 60 minutes, evaporate the ethanol to dryness, and vacuum-dry at 50°C for 10 hours to obtain puerarin phospholipids. The compound was ground through a 120-mesh sieve; 200g of the puerarin phospholipid compound was mixed with 400g of micropowdered silica gel, 1800g of lactose, and 800g of starch, and 300g of 50% ethanol and 150g of polysorbate 80 were taken and added to the mixture after mixing. Mix evenly, then use 300g of 70% syrup as a binder to make soft materials, pass through a 20-mesh sieve to make granules, dry at 55°C for 6 hours, pass through a 20-mesh sieve for granulation, and measure the content of puerarin in the granules by HPLC. Talcum powder 4g, pack and seal puerarin granules by containing 0.1g puerarin in every bag.
Embodiment 3
[0028] Embodiment 3: puerarin granules
[0029] Put puerarin and soybean lecithin in a ratio of 1:2 by weight, and dissolve them in 20 times absolute ethanol under the condition of reflux and stirring at 50°C. After reacting for 60 minutes, evaporate the ethanol to dryness, and vacuum-dry at 50°C for 10 hours to obtain the puerarin-phospholipid compound Grind and pass through a 120-mesh sieve; take 300g of puerarin phospholipid complex, mix with 500g of micropowder silica gel, 2000g of lactose, and 800g of starch, and then take 800g of 50% ethanol and 250g of polysorbate 80, mix them, and add them to the above mixture. , then use 400g of 70% syrup as a soft material for binder system, pass through a 20-mesh sieve to make granules, dry at 55°C for 6 hours, pass through a 20-mesh sieve for granulation, measure the content of puerarin in the granules by HPLC, add talcum powder 10g, packed and sealed with 0.1g puerarin per bag to obtain puerarin granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com