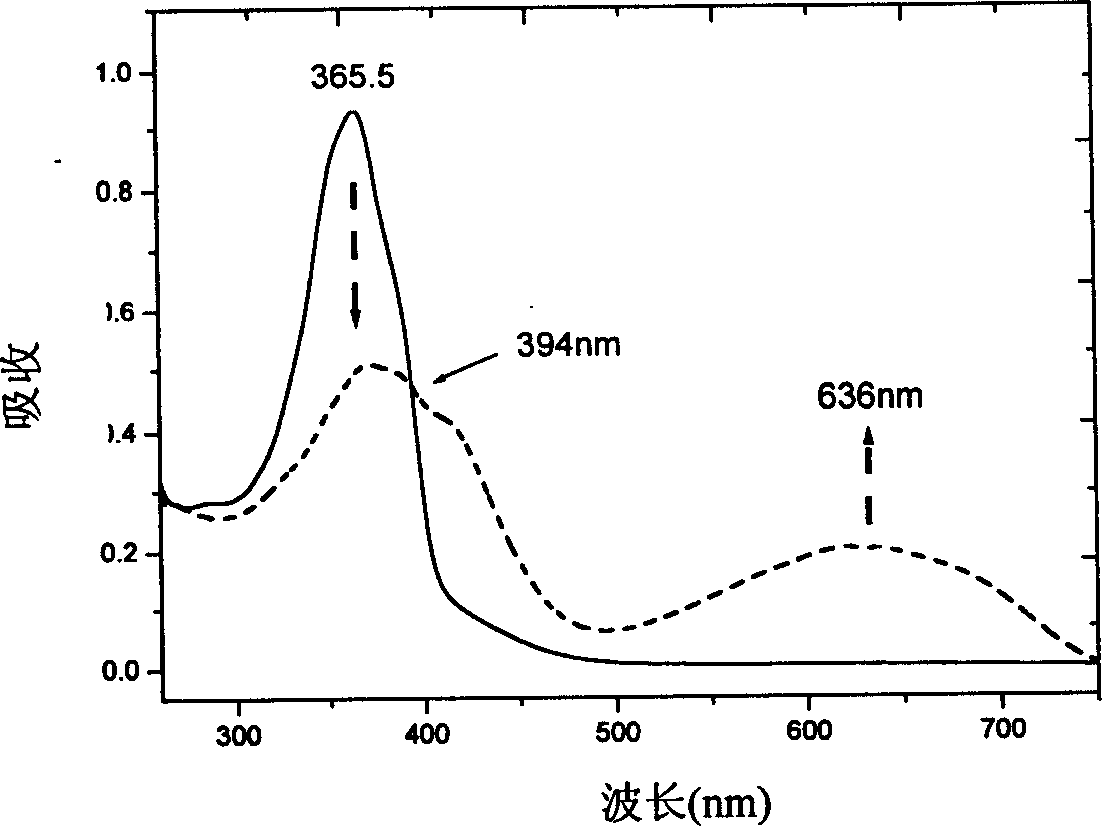
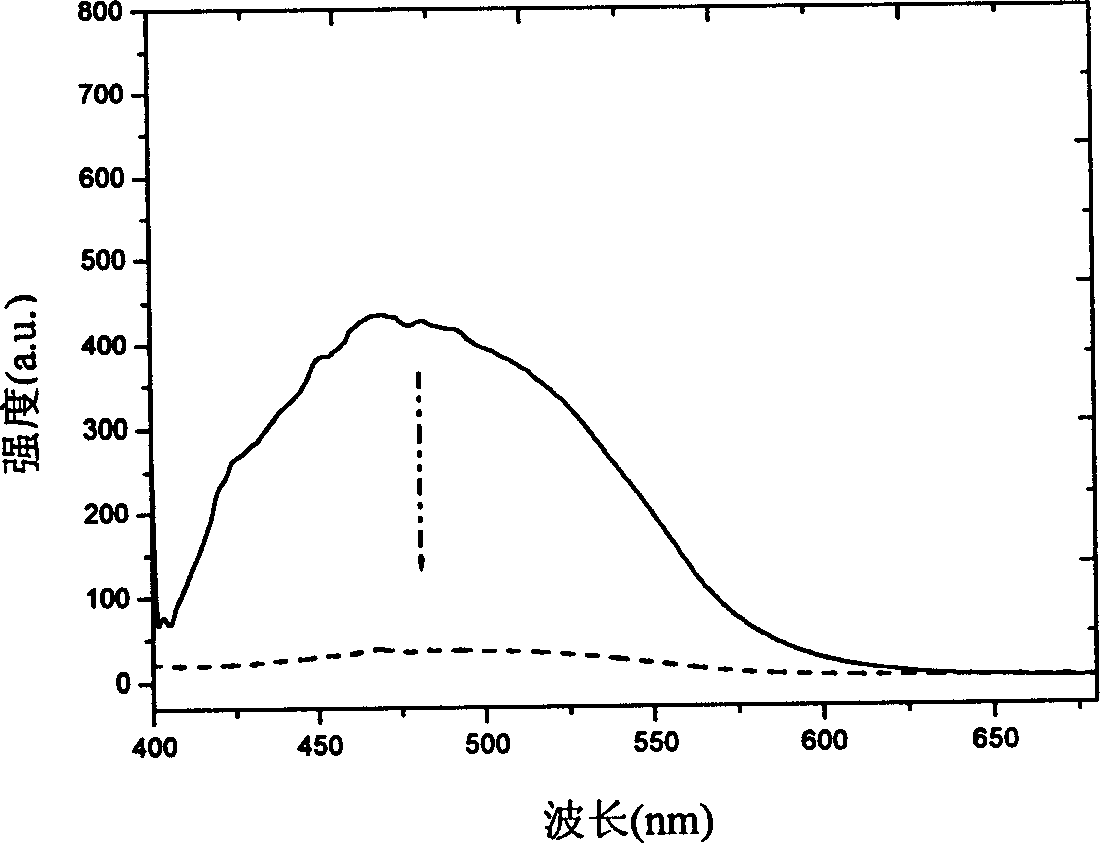
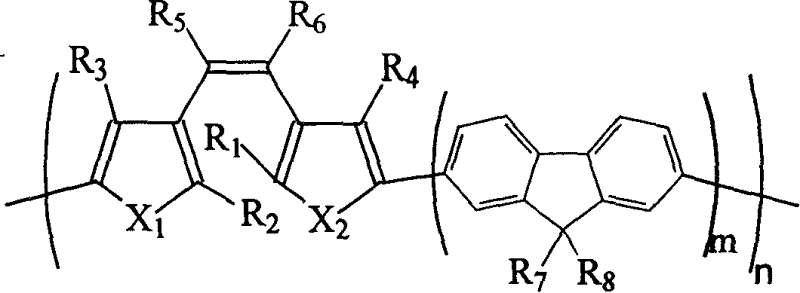
Dithiophene ethene functional material, and its preparing method and use
An aromatic heterocycle and compound technology, which is applied in the field of diarylethylene photochromic materials, can solve the problems of reducing the quantum efficiency of photochromic materials and affecting the performance of materials, and achieves high quantum efficiency, high degree of polymerization, and film-forming properties. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation method of embodiment 1 target polymer A:
[0034] Mix and dissolve halogenated dithiophene-substituted maleic dinitrile and 2,7-diboronic acid ethyl ester-9,9'-dioctylfluorene in a mass ratio of 1:2 in dry solvent tetrahydrofuran or toluene, add 2.5M Concentration of Na 2 CO 3 130 ml of aqueous solution and catalyst triphenylphosphopalladium (2‰ of the mass of halodithiophene substituted malonitrile). The whole reaction system is placed under the protection of inert gas. At 80°C, the reaction was stirred for 8 hours. After the reaction was completed, the mother liquor was cooled to room temperature, poured into a stirred mixed solvent of organic solvent methanol and deionized water (the weight ratio of the two was 8:1), and filtered to obtain a solid product. After washing the product repeatedly with water or methanol, Soxhlet extraction for one day, and vacuum drying to obtain the final product. The yield is about 68%.
Embodiment 2
[0035] The synthesis of embodiment 2 target object B:
[0036] In a 100mL one-necked flask, add ethyl bisborate substituted-dioctylbisdifluorene, 1,2-methyl-1,2-bis(2-methyl-5-halo-3-thiophene)-ethylene 10 mmol, THF was used as the solvent for the reaction (150 ml), and 0.03 mmol of catalyst was added and 50 ml of 2M NaOH aqueous solution was added. Argon vacuum replaced the system three times, and refluxed overnight in an argon atmosphere in the dark. After the reaction is completed, the mother liquor is poured into a mixed solvent of methanol and deionized water, filtered, washed with water and methanol respectively, subjected to Soxhlet extraction for 24 hours, and vacuum-dried to obtain the desired product.
Embodiment 3
[0037] The preparation of embodiment 3 target polymer C:
[0038] Mix and dissolve 5 mmol of brominated diarylmaleic dinitrile and ethyl bisborate substituted-dioctyl tertiary fluorene in a ratio of 1:1 in 120 ml of tetrahydrofuran solvent, add 30 ml of 2M NaOH aqueous solution and 2.5‰ (0.1 mmol) catalyst triphenylphosphopalladium. The reaction system was placed under protection from light and inert gas. The reaction was stirred at 60°C for 72 hours. After the reaction was completed, the mother liquor was cooled to room temperature, poured into a stirred mixed solvent of organic solvent methanol and deionized water (ratio: 10:1), and filtered to obtain a solid product. After washing the product with water or methanol, Soxhlet extraction for 36 hours, and vacuum drying to obtain the final product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com