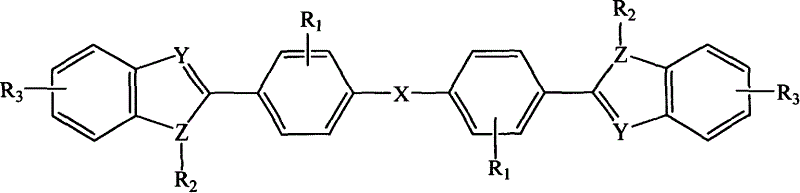
Diphenyl ether azole derivative bioelectronic transmission material, and its new preparing method and use
A technology of electron transport materials and azole derivatives, which can be used in light-emitting materials, chemical instruments and methods, organic chemistry, etc., and can solve problems such as shortage of materials that emit blue light.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
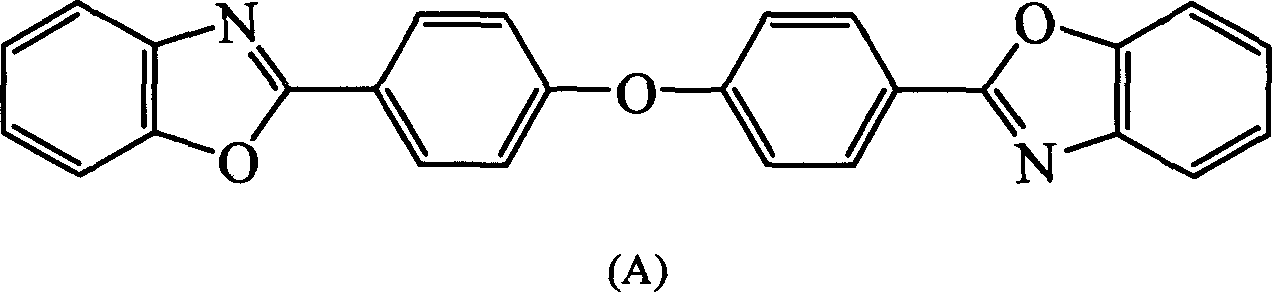
[0104] In a three-necked flask equipped with a stirrer, a nitrogen inlet tube, and a reflux condenser, add o-aminophenol (24mmol) and anhydrous tetrahydrofuran (THF) (30ml) in sequence, stir, and add 4,4'-oxygen in 50 minutes. Heterodibenzoyl chloride (10mmol), reacted at room temperature for 4 hours, then poured the reaction solution into 150ml deionized water, a white solid was precipitated, filtered, dried, and washed with THF / H 2 O was recrystallized to give white crystals 4,4″-oxabis[2′-hydroxy-benzoanilide]. Put 4,4″-oxabis[2′-hydroxy-benzoanilide] into the quartz tube , evacuated to 1 mmHg, heated to 250-270 ° C for 4 hours, cooled, and recrystallized with toluene to obtain 2,2'-(oxabi-p-phenylene)bisbenzoxazole, the compound ( A), the yield is 85%, and the melting point is 246-247°C.
Embodiment 2
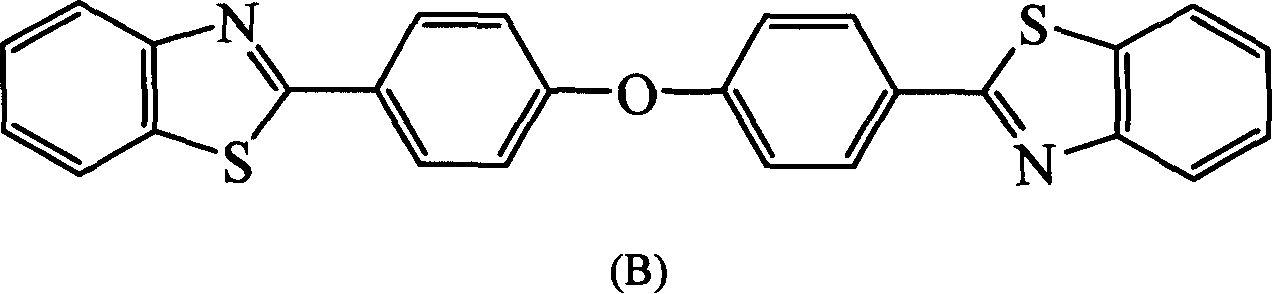
[0106] In a three-necked flask equipped with a stirrer, a nitrogen inlet tube, and a reflux condenser, add o-aminothiophenol (22mmol), 1,4-dioxane (30ml) successively, stir, and add 4 in 30 minutes, 4'-Oxadibenzoyl chloride (10mmol), reacted at room temperature for 8 hours, then poured the reaction solution into 250ml deionized water, a white solid was precipitated, filtered, dried, and washed with 1,4-dioxane / h 2 O recrystallized to obtain white crystal 4,4″-oxabis[2′-mercapto-benzoanilide]. Put 4,4″-oxabis[2′-mercapto-benzoanilide] into the quartz tube , vacuumize to 1mmHg, heat to 260-280°C for 8 hours, cool, and recrystallize with xylene to obtain 2,2'-(oxabi-p-phenylene)bisbenzothiazole, the compound ( B), yield 80%, melting point>280°C.
Embodiment 3
[0108] In a three-necked flask equipped with a stirrer, a nitrogen inlet tube, and a reflux condenser, N-phenyl-1,2-phenylenediamine (20mmol), N,N-dimethylformamide (DMF) (30ml ), stirred, and added 4,4'-oxadibenzoyl chloride (10mmol) in 15 minutes, reacted for 1 hour at room temperature, then poured the reaction solution into 200ml deionized water, white solids were precipitated, filtered, and dried , with DMF / H 2 O was recrystallized to give 4,4'-oxabis[N-[2-(anilino)phenyl]benzamide as white crystals. Put 4,4'-oxabis[N-[2-(anilino)phenyl]benzamide into a quartz tube, evacuate to 1mmHg, heat to 280-300°C for 6 hours, cool, use for solid Sublimation purification yielded 2,2'-(oxabi-p-phenylene)bis(3-phenylbenzimidazole), compound (L), with a yield of 75% and a melting point of 237-239°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| photoluminescence | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com