Adjuvant of slow release agent used for eye and medicine containing said adjurant and its preparation method
A sustained-release preparation and ophthalmic technology, which is applied in the field of pharmaceutical compositions containing the excipient and its preparation, can solve the problems of poor controllability of preparation quality standards, difficulty in achieving uniform drug delivery, and unstable gel state, etc. The effect of small drug content, maintaining effective drug concentration, and reducing dosing frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
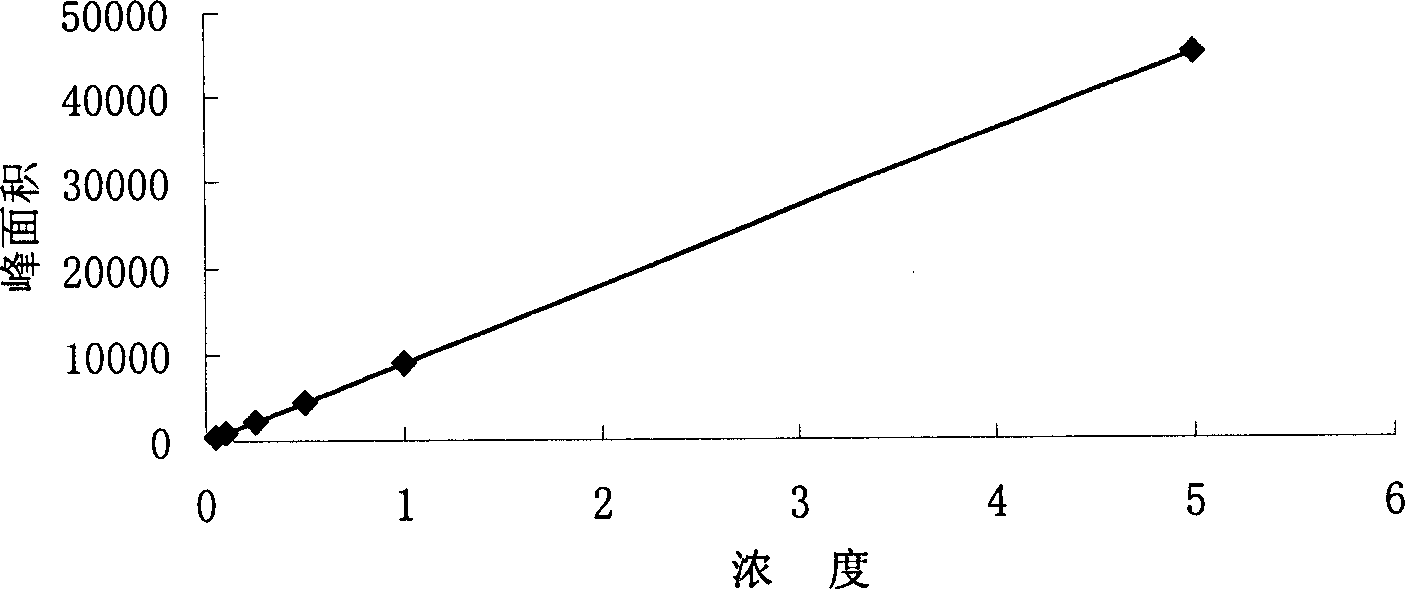
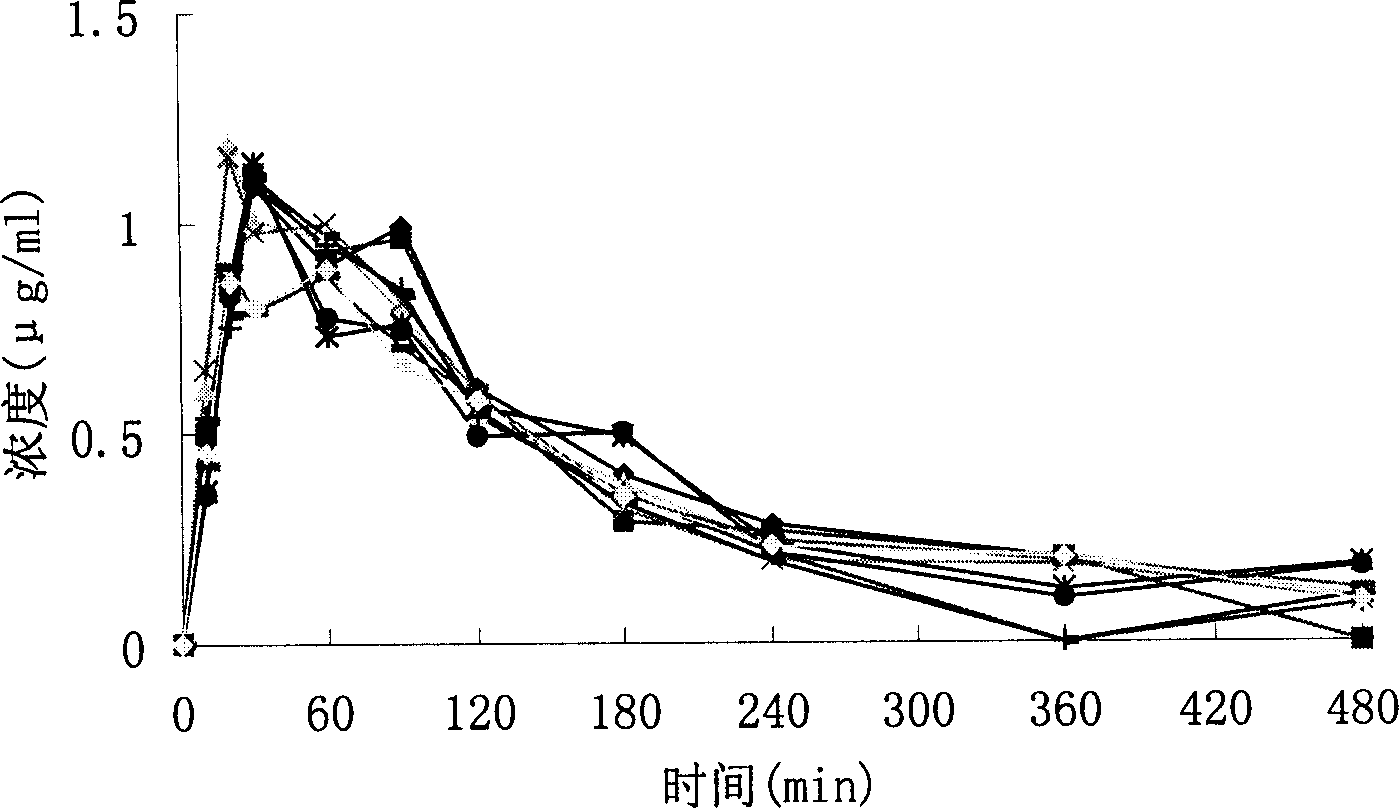
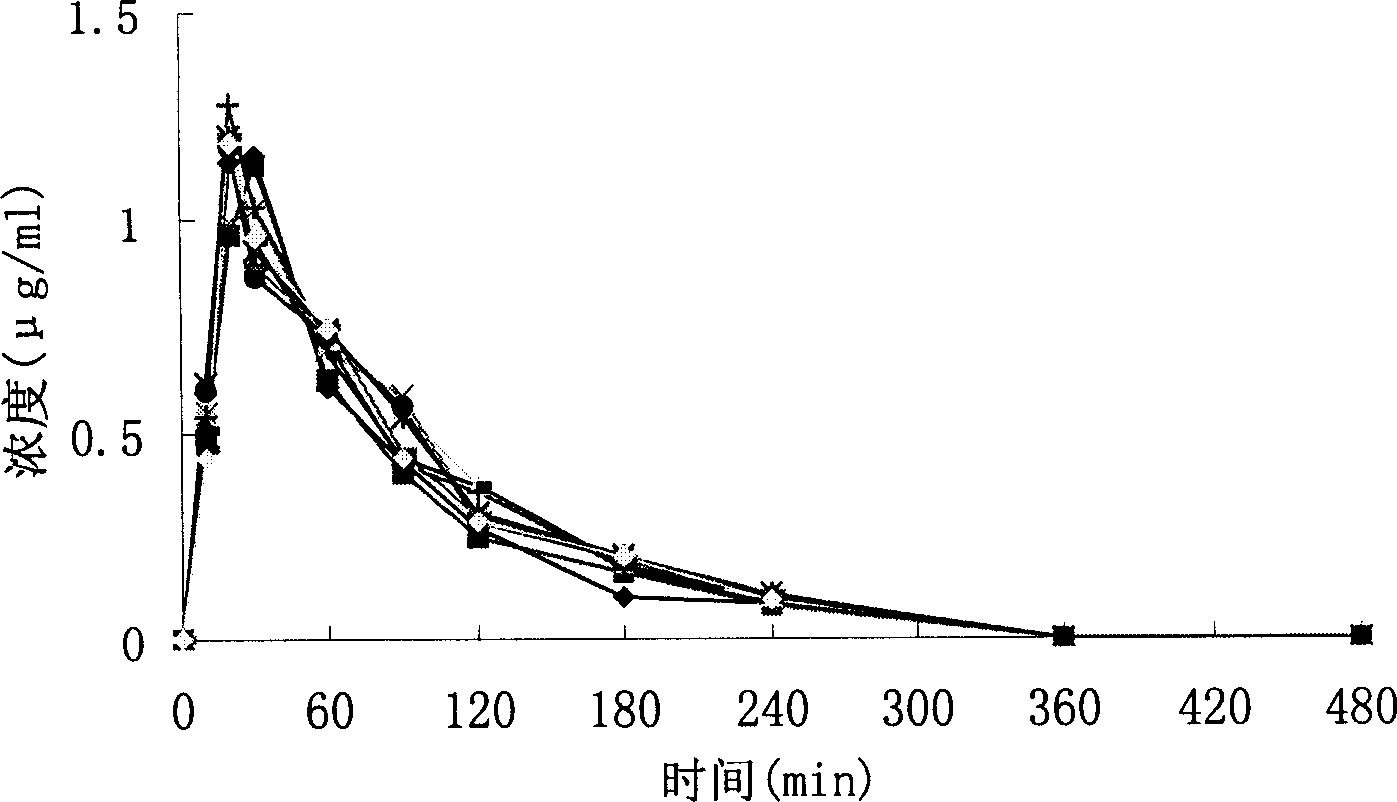
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 eye drops of the present invention
[0031] Raw materials: Levofloxacin Hydrochloride 5.0g, Carbomer 974P NF3.0g, Hypromellose E50LV 15.0g, Disodium Hydrogen Phosphate 2.5g, Sodium Dihydrogen Phosphate 5.0g, Sodium Chloride 5.0g, Parkin ethyl ester 0.3g, appropriate amount of 1M sodium hydroxide, water for injection to 1000ml;
[0032] step:
[0033] 1. Take disodium hydrogen phosphate, sodium dihydrogen phosphate and hydroxypropylmethyl cellulose, dissolve in 350ml water for injection, stir continuously to form a transparent solution, and set aside;
[0034] 2. Take carbomer and add it to 300ml water for injection to swell. After the swelling is complete, add it to the material in the above step 1, and keep stirring to form solution A;
[0035] 3. Add levofloxacin hydrochloride and sodium chloride to 100ml of water for injection, stir continuously, after dissolving, a light yellow transparent solution is formed, and set aside;
[0036...
Embodiment 2
[0039] Raw materials: Levofloxacin Hydrochloride 5.0g, Carbomer 974P NF1.0g, Hypromellose E50LV 15.0g, Disodium Hydrogen Phosphate 2.5g, Sodium Dihydrogen Phosphate 5.0g, Sodium Chloride 5.0g, Add 0.3 g of ethyl parkinate, an appropriate amount of 1M sodium hydroxide, and water for injection to 1000 ml; the steps are the same as in Example 1.
Embodiment 3
[0041]Raw materials: Levofloxacin Hydrochloride 5.0g, Carbomer 974P NF5.0g, Hypromellose E50LV 15.0g, Disodium Hydrogen Phosphate 2.5g, Sodium Dihydrogen Phosphate 5.0g, Sodium Chloride 5.0g, Add 0.3 g of ethyl parkinate, an appropriate amount of 1M sodium hydroxide, and water for injection to 1000 ml; the steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com