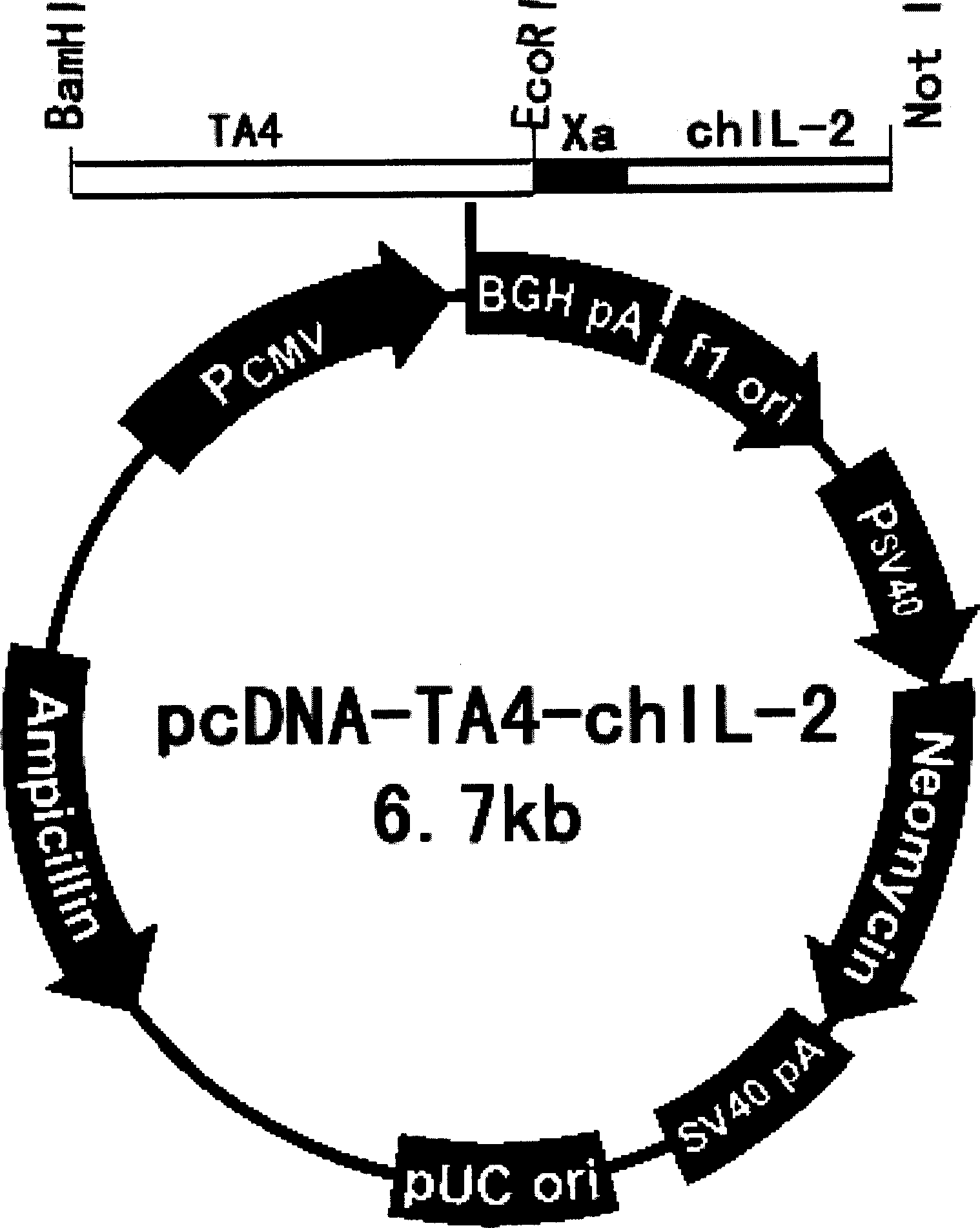
Immunity regulating type DNA vaccine for preventing and treating chicken coccidiosis
A DNA vaccine and immune regulation technology, applied in the field of DNA vaccines, can solve the problems of not being fully applicable to laying hens and broiler chickens, high cost, and difficult inoculation volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of embodiment 1.TA4 gene:
[0041] 1. Synthetic primers P1 and P2:
[0042] The following primers were designed according to the published nucleotide sequence of the TA4 gene:
[0043] P1: 5'- GGATCCG ATGAACAAGCTGA-3′
[0044] P2: 5'- GAATTC AAAGAGAGCGAAAGCGGA-3'
[0045] The underlined parts are the introduced enzyme cutting sites BamHI and EcoR I respectively.
[0046] 2. PCR amplification of TA4 gene
[0047] Add the following components to a thin-walled PCR tube for PCR amplification:
[0048] Plasmid pUC18-TA4 (50ng / μl) 1.0μl
[0049] 2.5mmol / L dNTP 4.0μl
[0050] P1 (50pmol) 1.0μl
[0051] P2 (50pmol) 1.0μl
[0052] 10×PCR buffer 5.0μl
[0053] 25mmol / L MgCl 2 23.0μl
[0054] Taq DNA polymerase (5U / μl) 0.5μl
[0055] wxya 2 O 34.5 μl
[0056] Total 50.0μl
[0057] After centrifuging and mixing the above components, denature at 95°C for 5 minutes on a PCR instrument; 30 cycles at 94°C for 60 seconds, 58°C for 60 sec...
Embodiment 2
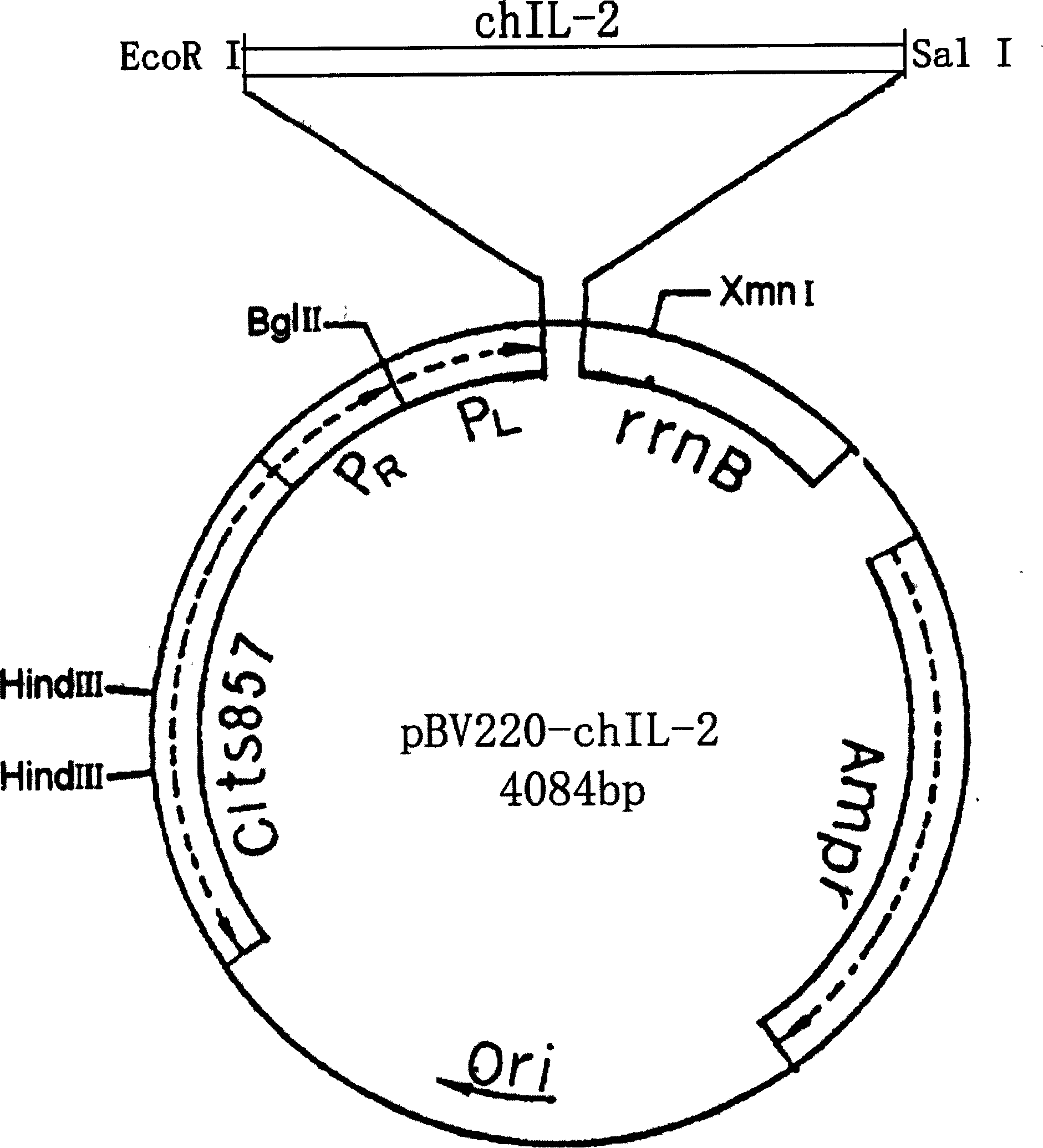
[0059] Example 2. Preparation of chIL-2 gene:
[0060] 1. Synthesize primers P3 and P4, the sequences are:
[0061] P3: 5'-CTA GAATTC CCTACCCTCGAT TGCAAAGTACTGATCT-3'
[0062] P4: 5'-TTA GTC GAC TTGCAGATATCTCCAAAAGTT-3'
[0063] The underlined part is the introduced enzyme cutting site EcoR I and SalI; the box part is the start codon; the italic letter part is the coagulation factor X a The specific hydrolysis sequence encodes a nucleotide sequence (such as the nucleotide sequence shown at positions 649-666 in SEQ ID NO: 1).
[0064] 2. PCR amplification of chIL-2 gene
[0065] Add the following components to a thin-walled PCR tube for PCR amplification:
[0066] Plasmid pBV220-chIL-2 (50ng / μl) 1.0μl
[0067] 2.5mmol / L dNTP 4.0μl
[0068] P3 (50pmol) 1.0μl
[0069] P4 (50pmol) 1.0μl
[0070] 10×PCR buffer 5.0μl
[0071] 25mmol / L MgCl 2 23.0μl
[0072] Taq DNA polymerase (5U / μl) 0.5μl
[0073] wxya 2 O 34.5 μl
[0074] Total 50.0μl
[0...
Embodiment 3
[0078] The construction of embodiment 3.DNA vaccine
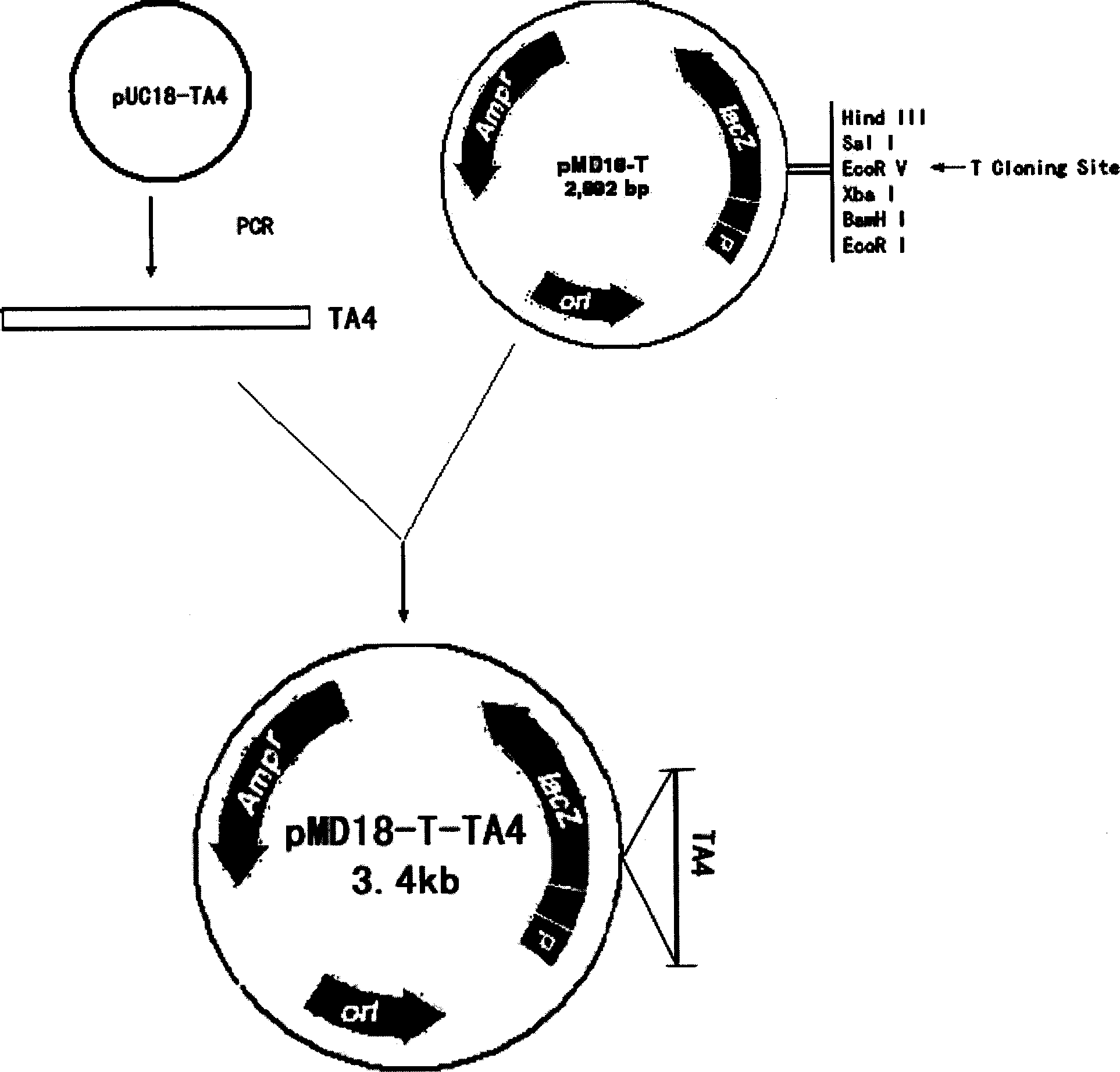
[0079] 1. Construction of recombinant plasmid pMD 18-T-TA4 (see image 3 )
[0080]Get 50 μ l of the PCR product obtained in Example 1, electrophoresis on 1% agarose gel, cut out the agarose gel at the band of interest under ultraviolet light, reclaim and purify the fragment of interest with the gel recovery kit of Dalian Bao Biological Company, method Refer to the instruction manual. Take the purified PCR product and connect it to the pMD18-T vector, and the reaction system is as follows:
[0081] PCR recovery product 4.0μl
[0082] pMD18-T vector 1.0μl
[0083] Ligation solution I 5.0μl
[0084] Total volume 10.0μl
[0085] The above components were mixed in a thin-walled eppendorf tube and connected overnight at 4°C. The ligation product was transformed into competent Escherichia coli JM109, and the plasmid was extracted, identified by double enzyme digestion with BamHI and EcoRI and sequenced, and determined to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com