Method for preparing methylal using combined continuous distillation and liquid-liquid extraction
A technology of reactive distillation and methylal, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of long production cycle, low yield, complex process, etc., and achieve short reaction cycle and high yield. High rate and high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
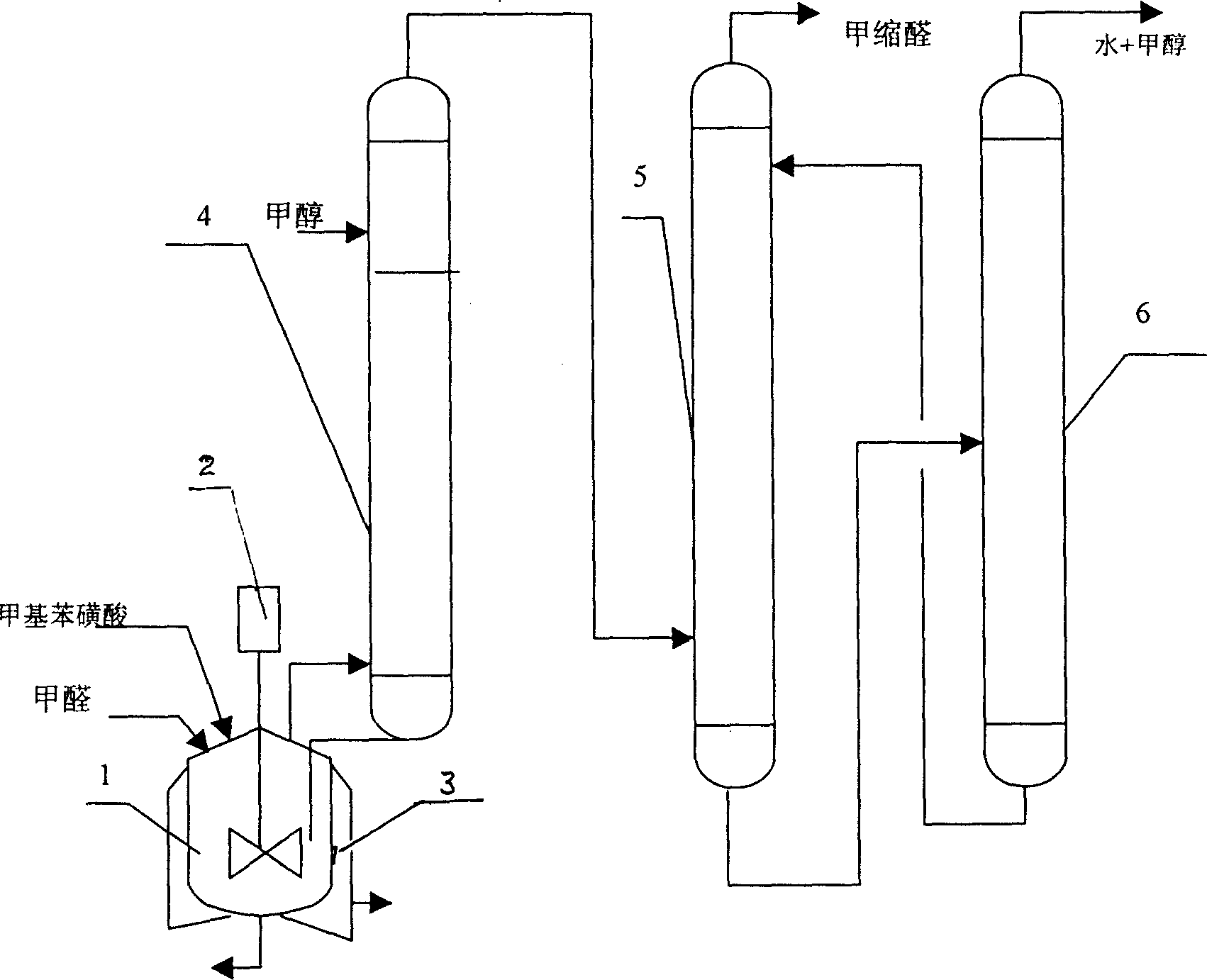
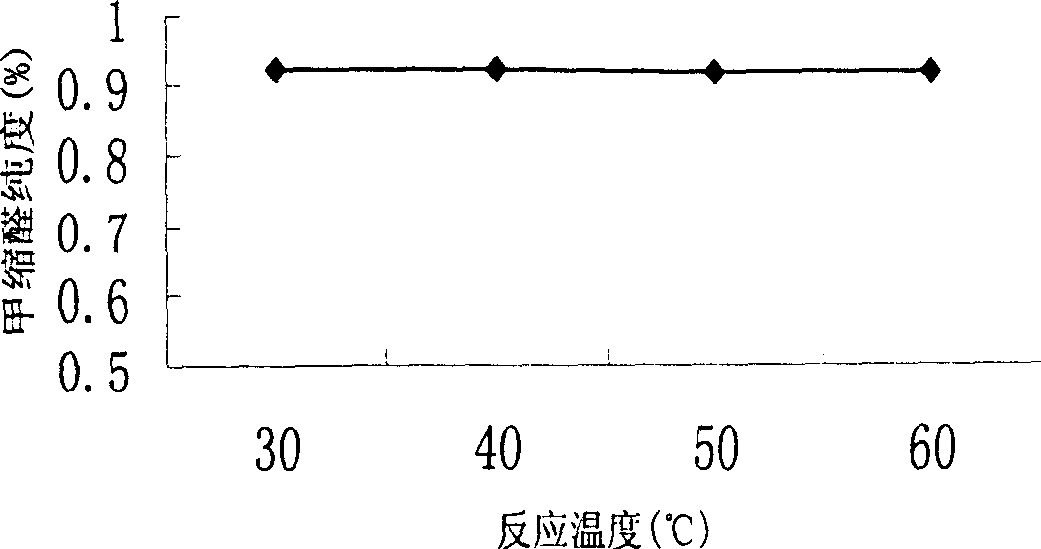
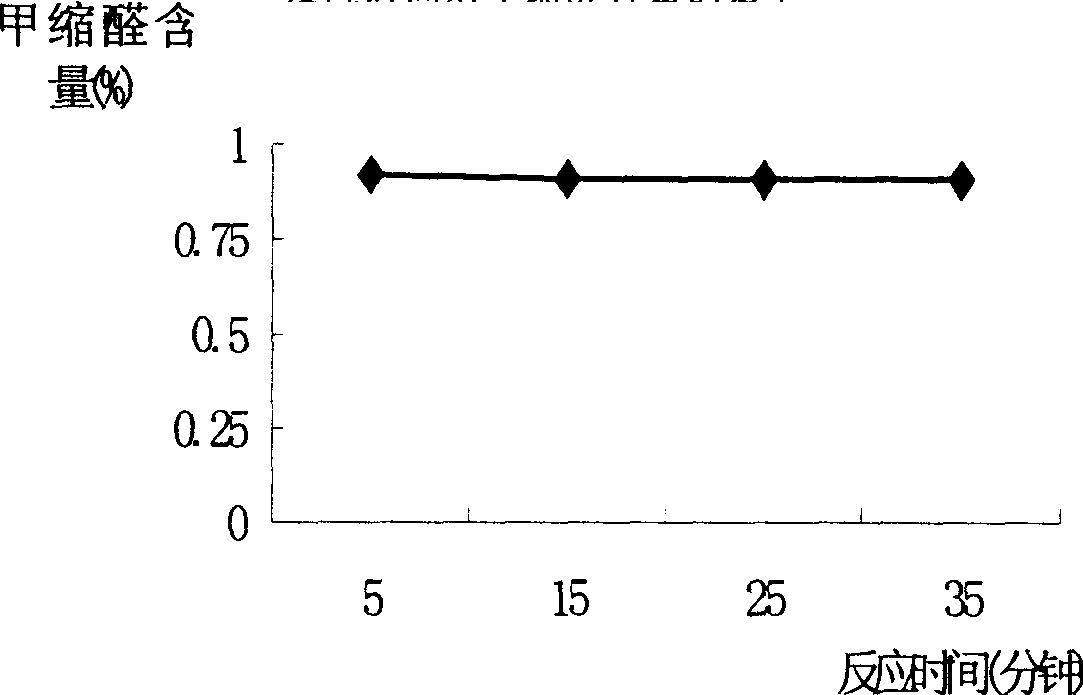
[0022] Embodiment 1, continuous reaction rectification and liquid-liquid extraction combine to prepare methylal process, with reference to Fig. 1: 37~40% formaldehyde presses the speed of 68ml / min, toluenesulfonic acid catalyst presses 5.5% of reaction mass total amount (mass ratio) is added in reactor 1, and 99.5% methanol is added from the 5th piece of rectification column 4 (feed position can be adjusted), and adding V / V is 101ml / min, after reaction rectification, tower top must distill The liquid contains relatively high methylal, methanol and water, and glycerol is used as the extraction agent, and the liquid-liquid extraction tower 5 extracts, and the methylal content at the top of the extraction tower can reach more than 99.7%. The extractant and the methanol-water mixture are recycled through the regeneration tower 6 for recycling. The methanol and water at the top of the regenerated tower can be further recycled and processed. The temperature of the reactor and top o...
Embodiment 2
[0023] Example 2 is basically the same as Example 1, but the catalyst is changed to sulfuric acid, and the extractant is changed to dimethylamine; the methanol / formaldehyde volume flow ratio is 1.4:1.
Embodiment 3
[0024] Embodiment 3 is substantially the same as Embodiment 1, but the methanol / formaldehyde volume flow ratio is 1.6:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com