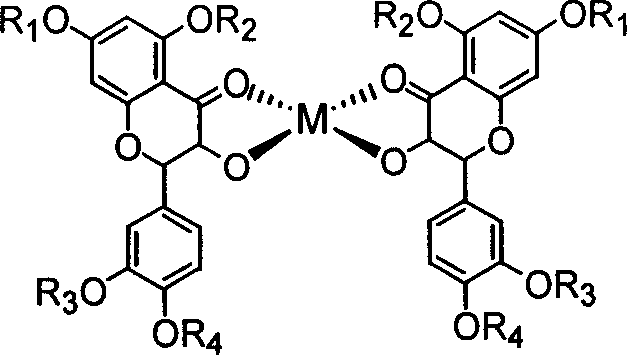
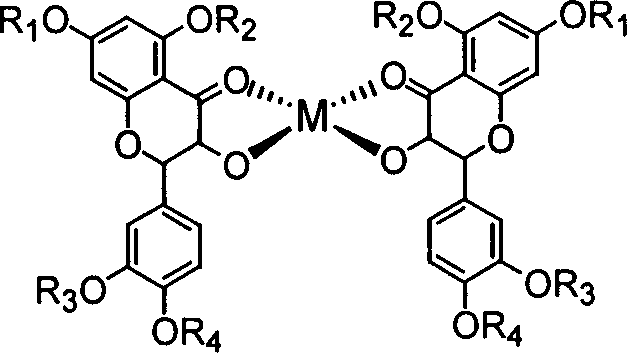
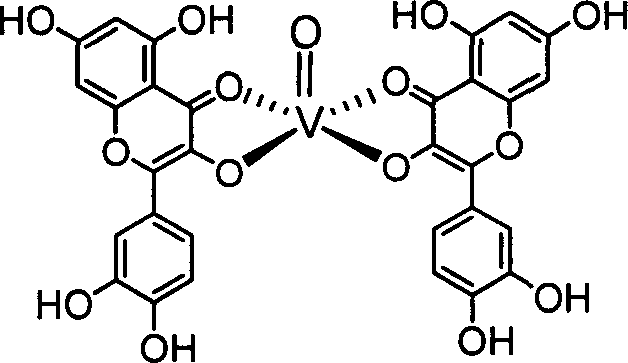
Compounds like quercetol and metal complex of their glycosides and uses
The technology of metal complexes and compounds is applied in the application field of preparing medicines for the treatment of diabetes, can solve the problems of low bioavailability, low activity, large toxic and side effects, etc., achieve good α-glucosidase inhibitory activity, improve diabetes Complications, the effect of reducing postprandial hyperglycemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Synthesis of quercetin vanadyl complex
[0018] Dissolve 10mmol quercetin in 70mL absolute ethanol solution, add 5mmol VOSO dropwise 4 ·3H 2 The aqueous solution of O was heated to 60° C., refluxed and stirred for 12 hours. After cooling to room temperature, an appropriate amount of water was added and allowed to stand for 30 hours. After suction filtration, the precipitate was washed several times with ethanol and water respectively, and then vacuum dried for 48 hours to obtain a dark blue powdery solid product.
[0019] Product structure:
[0020]
[0021] Product structure analysis data:
[0022] 1 HNMR(300MHz, DMSO-d 6 )δ: 11.60(1H,5-OH); δ10.74(1H,7-OH); δ9.32(1H,4′-OH); δ9.25(1H,3′-OH); δ7.93 (1H, H-2'); δ7.12(1H, H-6'); δ6.71(1H, H-5'); δ6.54(1H, H-8); δ6.31(1H, H-6); δ3.0~3.6(HO). m / z: 753.1 (M+).
[0023] The synthesis methods of other quercetin compounds and their glycoside vanadyl complexes are basically the same as the above methods.
Embodiment 2
[0024] Example 2: Synthesis of quercetin zinc complex
[0025] 5mmol zinc acetate was added to 70mL anhydrous ethanol solution containing 10mmol quercetin and an appropriate amount of sodium acetate, and the reaction was stirred under reflux for 10 hours, and a yellow flocculent precipitate was formed. After cooling to room temperature, add appropriate amount of water, leave it for 24 hours, and filter with suction. After washing the precipitate several times with ethanol and water respectively, it was dried in vacuum for 48 hours to obtain a yellow powdery solid product.
[0026] Product structure:
[0027]
[0028] Product structure analysis data:
[0029] 1 HNMR(300MHz, DMSO-d 6 )δ: 11.63(1H,5-OH); δ10.74(1H,7-OH); δ9.32(1H,4′-OH); δ9.25(1H,3′-OH); δ7.93 (1H, H-2'); δ7.12(1H, H-6'); δ6.71(1H, H-5'); δ6.54(1H, H-8); δ6.31(1H, H-6); δ3.0~3.6(HO). m / z: 690.1 (M+).
[0030] The synthesis methods of other quercetin compounds and their glycoside zinc complexes are basically the sam...
Embodiment 3
[0031] Example 3: Synthesis of copper quercetin complex
[0032] Add 5mmol of copper acetate to 70mL of absolute ethanol solution containing 10mmol of quercetin and an appropriate amount of sodium acetate, reflux and stir the reaction for 10 hours, a green flocculent precipitate is produced. After cooling to room temperature, add an appropriate amount of water and leave it for 48 hours. Suction filtration. After washing the precipitate several times with ethanol and water respectively, it was vacuum dried for 40 hours to obtain a green powdery solid product.
[0033] Product structure:
[0034]
[0035] Product structure analysis data:
[0036] 1 HNMR(300MHz, DMSO-d 6 )δ: 11.63(1H,5-OH); δ10.74(1H,7-OH); δ9.32(1H,4′-OH); δ9.25(1H,3′-OH); δ7.93 (1H, H-2'); δ7.12(1H, H-6'); δ6.71(1H, H-5'); δ6.54(1H, H-8); δ6.31(1H, H-6); δ3.0~3.6(HO). m / z: 716.1 (M+).
[0037] The synthesis methods of other quercetin compounds and their glycoside zinc complexes are basically the same as the above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com