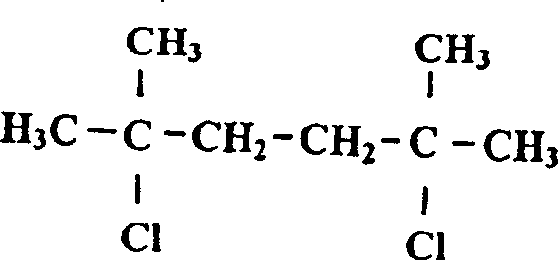Method synthesizing arotinoid acid and arotinoid ethylester, and its pharmaceutical application
A technique of aromatic ethyl retinoic acid and a synthetic method, which is applied in the synthetic method and the application field in pharmacy, can solve problems such as not fully and specifically disclosing the application, and achieve the effects of cost reduction, good product quality, and shortened time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
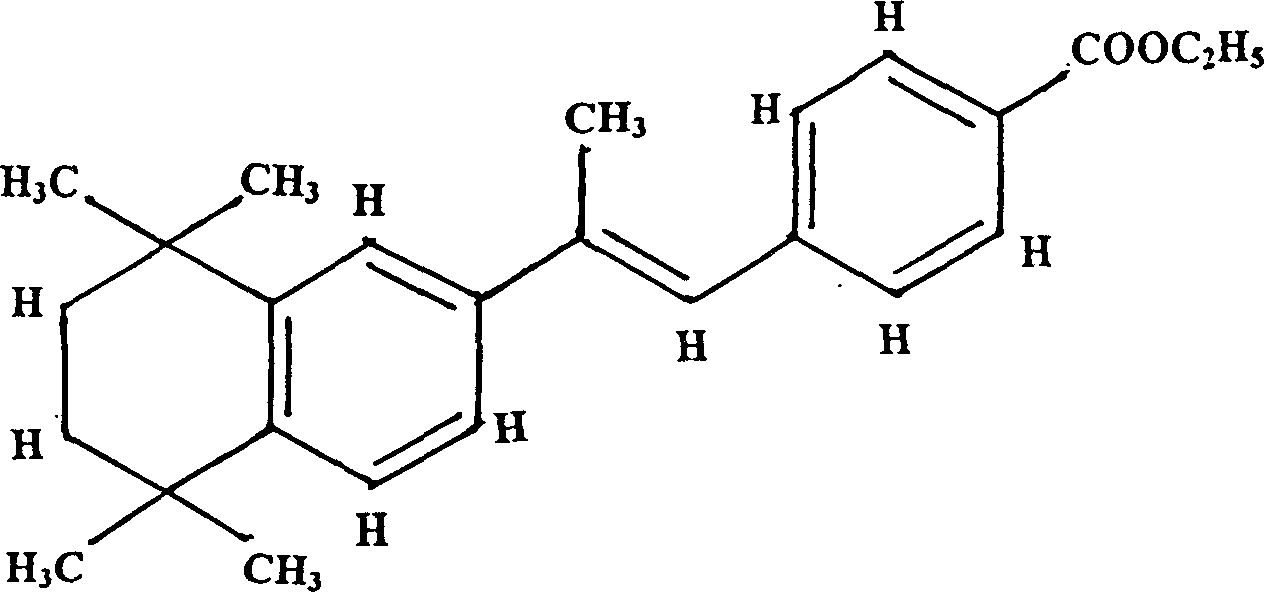
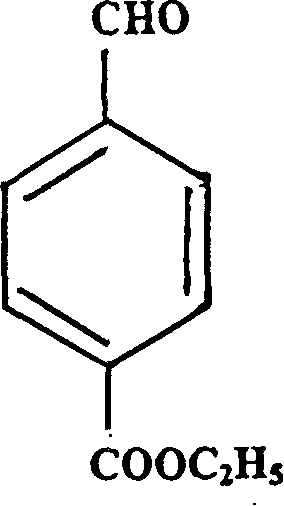
[0057] Example 1: Synthesis of aromatic ethyl retinoate, namely P-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl) propenyl]-ethyl benzoate.
[0058] The first step, preparation [1-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)-ethyl]-triphenylphosphine bromide, its step as follows:
[0059] a, Preparation of 2,5-dimethylhexanedichloro-2,5
[0060] Add 365g of 2,5-dimethylhexanediol-2,5 into 2500ml of concentrated hydrochloric acid, let it stand at room temperature for 24 hours, separate the solid, wash it with water to remove the acid, wash it twice with methanol, and dry it The measured melting point is 64-65°C, which is 2,5-dimethylhexanedichloro-2,5;
[0061] b, preparation of 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-naphthalene
[0062] Add 73.2g of 2,5-dimethylhexanedichloro-2,5 into 500ml of benzene, then add 10.5g of aluminum trichloride, heat to 60-80°C, reflux for 6 hours, remove benzene, collect 90 ~105℃ / -0.093MPa fraction, the measured refractive ...
Embodiment 2
[0079] Embodiment two. synthetic aromatic retinoic acid, its steps are as follows:
[0080] The aryl retinoic acid ethyl ester synthesized in Example 1, that is, P-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl ) propenyl]-ethyl benzoate 50g was added in 1100 milliliters of ethanol, then 500 milliliters of 0.5N NaOH was added, and refluxed for 2.5 hours;
[0081] Use 0.5N sulfuric acid to adjust the pH value to 1-2, separate the solid, and wash it with water to remove the acidity;
[0082] Reflux with dichloromethane to dissolve, recover and remove most of the dichloromethane, add n-hexane to the remaining solution, and precipitate a solid, which is P-[(E)-2-(5,6,7,8-tetrahydro-5 , 5,8,8-tetramethyl-2-naphthyl)propenyl]-benzoic acid, referred to as aromatic retinoic acid or aromatic retinoic acid (Arotinoic acid), melting point 248 ~ 249 ° C.
Embodiment 3
[0083] Embodiment 3. Fragrant ethyl retinoic acid capsules, each containing 0.03 mg of aromatic ethyl retinoic acid and 200 mg of starch; combined with "8-methoxy psoralen long-wave ultraviolet photochemical therapy" for the treatment of psoriasis, "8 -Methoxypsoralen long-wave ultraviolet photochemical therapy" According to conventional treatment, the patient took the aromatic ethyl retinoate capsule once a day, 1 capsule each time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com